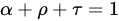Infrared (IR) technology is highly energy-efficient, less water-consuming, and environmentally friendly compared to conventional heating. Further, it is also characterized by homogeneity of heating, high heat transfer rate, low heating time, low energy consumption, improved product quality, and food safety. Infrared technology is used in many food manufacturing processes, such as drying, boiling, heating, peeling, polyphenol recovery, freeze-drying, antioxidant recovery, microbiological inhibition, sterilization grains, bread, roasting of food, manufacture of juices, and cooking food.
1. Introduction
Heating is one of the important thermal processes in food processing and depends on the transfer of heat by conduction, convection, and radiation
[1]. The goal of heating food is to increase its shelf life
[2]. Traditional heating is either by burning fuel or by using electrical source. Heat is transferred from the outside to the surface of the food through convection and conduction and the temperature of the surface of the food increases even more until the heat reaches through the conduction into the food. When the temperature increases more, it causes physical and chemical changes of the heated substance
[3].
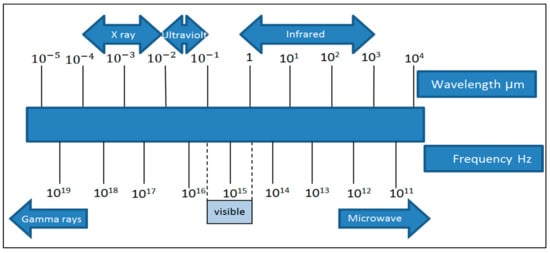
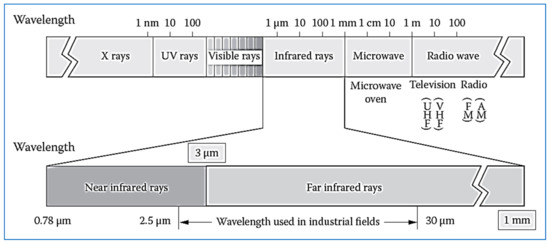
Infrared is part of the electromagnetic spectrum that is located between the visible region and microwaves, and its wavelength ranges from 0.5 to 100 µm. Infrared rays are divided into three types: Near-IR (NIR) whose wavelength ranges from 0.75 to 1.4 µm at temperatures below 400 °C, and mid-IR (MIR) whose wavelength is between 1.4 and 3 µm at temperatures between 400 and 1000 °C and far-IR radiation (FIR) wavelength of 3–1000 µm at temperatures above 1000 °C
[4][5][4,5]. Infrared penetration causes vibrating movement of water molecules at a frequency of 60,000–150,000 MHz and thereby causes heating. The dissipation of radiant energy in the form of heat produces a warming of the surface of the food and the penetration depth depends on the thickness of the product, the water activity, the product components and the wavelength of the infrared radiation
[4]. Pan and Atungulu
[6] described the benefits of infrared technology. Infrared technology is characterized by high energy efficiency, less water consumption, and environmental friendliness. In addition, it is also characterized by the homogeneity of heating with high heat transfer rate, low heating time, low energy consumption, and improving product quality and food safety. It also features low energy costs and the size of infrared equipment is small as well as controlling factors with high accuracy
[7]. Moreover, it possesses unique radiation properties. The thermal efficiency of IR is high, and it is considered a valuable energy source. Gary Zeman who is a certified health physicist and the spokesman for the Health Physics Society pointed out that based on a study conducted by the National Research Council, there was no link between infrared cooking and cancer in an interview with The Jamaican Gleaner in 2008. The study also indicated that infrared radiation did not have enough energy to halve or damage DNA
[8]. The energy is directly concentrated on the material to be heated and does not produce volatile organic compounds, carbon monoxide, or nitrogen oxides. It does not need heat recycling and does not need an isolated system. Nindo and Mwithiga
[9] stated that infrared radiation does not pollute the environment compared to fossil fuels. In addition, it preserves vitamins, has very little flavor loss, and no heating of the surrounding air
[5][10][11][5,10,11]. The Food and Drug Administration (FDA) has indicated that infrared radiation can be used in food processing, which is not ionizing. However, Mortensen
[8] stated that care must be taken when using infrared radiation because it produces high heat, causes burns, low penetration depth of foods, and long-term exposure to infrared radiation causes tissue rupture
[2].
Numerous studies have indicated that infrared possessed efficient effects in many manufacturing processes. Anagnostopoulou et al.
[12] found that the total phenols found in infrared dried orange peel were higher compared to the dried orange peel using hot air. Many researchers have reported that infrared radiation improves the quality of food
[13][14][13,14] and inhibits enzymes
[15]. Infrared was used in the bakery because of its high efficiency in heat transfer processes
[16]. In order to further increase its efficiency and improve energy use, microwave and infrared radiation were used together in roasting hazelnuts
[17]. In addition, infrared radiation can be used to heat liquid food such as lemon juice
[18].
Infrared can be used in numerous ways, such as stabilization of immature rice grain
[19], pre-heating of drying
[20][21][22][20,21,22], peeling
[23], polyphenol recovery
[24], freeze-drying
[25], refractance window drying
[26], antioxidant development
[27], microbiological inhibition
[28], sterilization infrared grains
[29], baking bread
[30], roasting food
[31], manufacture of juices
[18][32][18,32], and cooked food
[33][34][33,34].
2. Infrared Radiation
Infrared radiation is part of the electromagnetic spectrum that is located between the visible region and microwaves. Its wavelength ranges from 0.5 to 100 µm. In 1800, Herschel wrote the first scientific report on infrared heating. In his report, it was shown that when the lenses were exposed to the sun, the paper beneath was burned. In the study, a prism was used to separate the light into different colors and a thermometer was subsequently placed in the different colors of the solar energy spectrum. Researchers noticed that there was severe heating in the area beyond the red color, which began at the end of the visible spectrum. The invisible part of the spectrum is called infrared and it is in the wavelength range of 0.76–350 µm
[3]. The heat from the sun’s rays is infrared and is part of the electromagnetic radiation
[6]. Sixty-six percent of solar radiation is infrared.
Figure 1 and
Figure 2 illustrate the electromagnetic spectrum of different types of radiation, including infrared.
Figure 1.
Electromagnetic radiation spectrum.
Figure 2.
Spectrum of electromagnetic waves and infrared ranges.
The wavelength
λ is the inverted frequency
ν and can be calculated according to the following equation:
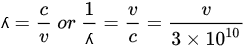
where
c is the speed of electromagnetic radiation (cm/s).
The wave number can be calculated from the following equation and its units (1/cm)
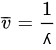 ΔE
ΔE can be calculated from the following relationship:
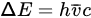
The conversion between wavelength and wavenumber is:
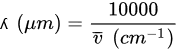
The maximum obtained power at a given wavelength µm and
T divided by the unit area (cm
2) of the black body is calculated as follows:
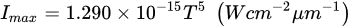
The Stefan–Boltzmann function shows the relationship between the intensity of the emitted radiation (
I) and the absolute temperature (
T) of the source as in the following equation:
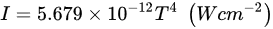
Infrared radiation is divided into three types
[3]:
-
Near-IR (NIR) with wavelength ranging from 0.75 to 1.4 µm.
-
Mid-IR (MIR) with a wavelength between 1.4 and 3 µm.
-
Far-IR radiation (FIR) with wavelength between 3 and 1000 µm.
Therefore, infrared radiation is defined as part of an electromagnetic spectrum whose wavelength ranges from 0.75 to 1000 µm.
Infrared heating depends on the spectrum because the energy emitted from the emitter consists of different wavelengths and part of the radiation depends on the source temperature and the lamp emission. The phenomenon of radiation becomes more complex because the amount of radiation that falls on any surface depends not only on the spectrum, also on the direction. Electromagnetic radiation is weakened as a result of absorption by the medium as well as scattering. The process of converting radiation to other forms of energy is a phenomenon of absorption, while in the case of scattering, the radiated energy is directed to another destination from the original direction of propagation as a result of the combined effect of reflection, refraction, and deviation, and all these factors cause weak electromagnetic radiation
[13][34][13,34].
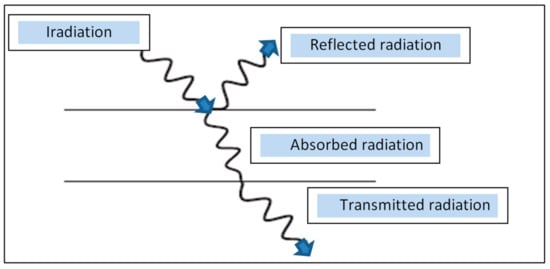
The radiation falling on a particular object is converted to heat and the energy can be absorbed and reflected, in addition, the radiation can be absorbed and transmitted as shown in
Figure 3. This figure shows that there are three basic radiation properties, they are reflectivity (
ρ), which is the ratio of the reflected part of the radiation coming to radiation next macro. Absorptivity (
α), which is the ratio of the absorbed portion of incoming radiation to total incoming radiation. Emissivity transmissivity (
τ), which is the ratio of the emitted part of the incoming radiation to the total incoming radiation, and the energy balance is shown in the following equation:
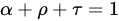
Figure 3.
Extinction of radiation [6].
3. Infrared Food Heating Mechanism
Energy conservation is one of the factors that determine the usefulness and success of the operation of any food industry unit. Heat is transmitted by conduction, convection, and radiation. The goal of heating food is to increase the shelf life and improve the taste of foods
[2]. Temperature is a measure of thermal motion at the molecular level. When the temperature of the material increases, the molecular motion gains more energy, and when it increases more, it causes physical and chemical changes in the heated material. In conventional heating, which comes from the combustion of fuel or electric heaters, heat is transferred to the material from the outside by convection by hot air or by thermal conduction. The process of transferring energy from source to food depends on the type of cooking. For example, in the case of the baking process, the energy is transmitted through convection, while frying and boiling are through conduction. Energy will be very close to the surface of the food and then heat food gradually from the hot surface towards the inside. Heat is transferred to the food through conduction only and this requires continuous processing of heat. The high temperature and time required for food depend on the thermal and engineering properties of the food
[3].
When heating is done by radiation, the heat is transferred by convection and conduction. The broiling process takes place due to thermal radiation. Electromagnetic radiation causes thermal movements of the molecules, but conversion efficiency is highly dependent on the frequency (energy) of the radiation. Radiation-transmitted energy at shorter wavelengths than infrared causes electron-chemical changes in radiation-absorbing molecules, such as chemical bonding, electronic excitation, and dissipation of absorbed energy in the form of less heat. The efficiency of converting absorbed energy into heat is great at high wavelengths in infrared radiation, so the electromagnetic radiation produced by infrared radiation deepens the food by a few millimeters. Infrared radiation is absorbed by organic matter at separate frequencies that correspond to the transport of internal molecules between energy levels. This transition within the range of infrared energy is expressed regarding the rotational movement and the vibrational (stretching) movement of internal atomic bonds. The rotational frequencies range from 1011 to 1013 Hz with a wavelength of 30 µm
−1 mm. The energy transfer during the separation of liquids is very small, and therefore, infrared absorption is continuous. Infrared absorption bands associated with food heating are shown in
Table 1.
Table 1. The infrared absorption bands characteristic of chemical groups relevant to the heating of food
[3].
|
Relevant Food Component
|
Absorption Wavelength (μm)
|
Chemical Group
|
|
Water, sugars
|
2.7–3.3
|
Hydroxyl group (O–H)
|
|
Lipids, sugars, proteins
|
3.25–3.7
|
Aliphatic carbon-hydrogen bond
|
|
Lipids
|
5.71–5.76
|
Carbonyl group (C=O) (ester)
|
|
Proteins
|
5.92
|
Carbonyl group (C=O) (amide)
|
|
Proteins
|
2.83–3.33
|
Nitrogen-hydrogen group (–NH–)
|
|
Unsaturated lipids
|
4.44–4.76
|
Carbon-carbon double bond (C=O)
|
Table 1 shows that there is a strong absorption due to longitudinal vibrations. The absorption of the material to the radiation does not make it saturated with infrared radiation because the molecules excited by the vibratory movement continuously lose energy in random directions as a result of collisions between the molecules, which transfer energy to the surrounding environment in the form of heat. Wavelengths ranging within 1.4–5 µm are considered more effective in cooking food because of their ability to penetrate the steam layer surrounding the food as well as within the food a few millimeters deep. Most infrared radiation is absorbed by a thin layer of organic matter and water, so heating is superficial. The process of infrared heating is faster because the energy is transferred from the heating source to the food simultaneously. Therefore, there is no need for another method to transfer energy, for example, the use of hot air. The heat from infrared heating is produced on the surface of the infrared treated material, so the inside of the material is heated by the connection between the food molecules, thus the temperature is graded from the surface to the center. The air in contact with the surface of the food is heated indirectly, but it is not as hot as it occurs in heating by convection and conduction.
The infrared absorption ranges by food components are shown in Figure 4, which shows that the food components interfere with each other in the absorption of different infrared spectra. Water mainly affects the absorption of incident radiation at all wavelengths, while the absorption of proteins by infrared radiation is at wavelengths 3–4 and 6–9 µm. Fat absorption is at wavelengths 3–4, 6 and 9–10 µm, and sugars are 3 and 7–10 µm. The water absorption beams are 3, 4.7, 6, and 15.3 µm
[13]. In addition, when the thickness of the food increases, the absorption increases.
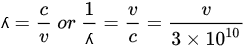 where c is the speed of electromagnetic radiation (cm/s).
The wave number can be calculated from the following equation and its units (1/cm)
where c is the speed of electromagnetic radiation (cm/s).
The wave number can be calculated from the following equation and its units (1/cm)
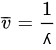 ΔE can be calculated from the following relationship:
ΔE can be calculated from the following relationship:
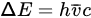 The conversion between wavelength and wavenumber is:
The conversion between wavelength and wavenumber is:
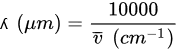 The maximum obtained power at a given wavelength µm and T divided by the unit area (cm2) of the black body is calculated as follows:
The maximum obtained power at a given wavelength µm and T divided by the unit area (cm2) of the black body is calculated as follows:
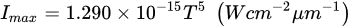 The Stefan–Boltzmann function shows the relationship between the intensity of the emitted radiation (I) and the absolute temperature (T) of the source as in the following equation:
The Stefan–Boltzmann function shows the relationship between the intensity of the emitted radiation (I) and the absolute temperature (T) of the source as in the following equation:
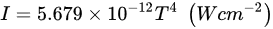 Infrared radiation is divided into three types [3]:
Infrared radiation is divided into three types [3]: