Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Teresa Poerio and Version 2 by Peter Tang.
The world plastic production is constantly growing, with production rising from 335 million tons in 2016 to 348 million tons in 2017. The significant and continuous increase in the production of plastics causes an enormous amount of plastic waste on the land entering the aquatic environment. Furthermore, wastewater treatment plants (WWTPs) are reported as the main source of microplastic and nanoplastic in the effluents, since they are not properly designed for this purpose. Among the tertiary treatment processes, membrane operations can offer an effective solution to the microplastic and nanoplastic pollution in the effluents.
- plastic removal
- wastewaters treatment
- membrane processes
- ultrafiltration
- dynamic membranes
- reverse osmosis
- membrane bioreactors
- membranes reuse
- membranes recycling
1. Introduction
The world plastic production is constantly growing, with production rising from 335 million tons in 2016 to 348 million tons in 2017. [1]. Asia is the largest producer of plastics (50.1%), followed by Europe (18.5%), North American Free Trade Agreement (17.7%), Middle East, Africa (7.71%), Latin America (4%) and Commonwealth of Independent States (2.6%). This significant increase and widespread in worldwide production of plastics produces a huge amount of plastics waste on land that enters the aquatic environment causing growing concerns [2].
Different papers report that the presence of a large part of microplastic fibers in the aquatic environment is due to the washing of synthetic clothes [3][4][3,4]. The ingestion of microplastic, besides causing the obstruction of digestive tract, can facilitate the transfer of contaminants adsorbed by the plastic, with unclear consequences to the health of aquatic organisms and humans [5][6][7][5,6,7]. Indeed a major problem with microplastic is their ability to adsorb other common environmental contaminants, such as metals [8][9][10][8,9,10], pharmaceuticals [11][12][11,12], personal care products [12] and others [13][14][13,14]. Consequently, the microplastic can potentially cause diseases such as cancer, a malformation in animals and humans, impaired reproductive activity, and reduced immune response [15].
Microplastic removal from the aquatic environment represents a new urgent challenge in the last decade considering the disastrous impact for aquatic species and human beings. These contaminants have been detected in various aquatic environments such as lakes, rivers, oceans, urban wastewater effluents.
Based on the particle size, plastics are defined as microplastic (MP) and nanoplastic (NP).
According to the National Oceanic and Atmospheric Administration (NOAA), the definition of microplastic is particles of synthetic polymers (less than 5 mm in diameter), which resist (bio) degradation [16]. On the other hand, nanoplastic is defined as particles (nanospheres, nanowires/nanotubes, and nanofilms) with smaller dimensions, between 1 and 100 nm [17][18][19][20][17,18,19,20].
Based on their origin, MP and NP are divided into two classes, primary and secondary plastic [21]. The first includes small pieces of specially manufactured plastic, such as hand and facial cleansers, shower gels, toothpaste, industrial scrubbers, and plastic micro-nanospheres, etc., while the latter are small pieces of plastic derived from the deterioration of larger plastic waste both at sea and on land.
Nowadays, 98% of MP is retained from wastewater treatment plants (WWTPs) but MP with a size smaller than 20 μm and NP is not retained; therefore, WWTPs plants are supposed to be one of the major responsibility for the plastic pollution in wastewater effluents [22][23][24][22,23,24]. The wastewater processing can be grouped into four main treatments: preliminary treatment, primary treatment, secondary treatment, and tertiary treatment, also named final or advanced treatment [25] (Figure 1).

Figure 1.
Classification of wastewater treatment methods.
2. Ultrafiltration
Ultrafiltration (UF) represents a feasible alternative for the water treatment since it permits to attain drinking water of high quality in an economic manner thanks to low energy consumption, high separation efficiency, and compact plant size [26][27][28][39,40,41]. It is a low-pressure process (1–10 bar) that, using asymmetric UF membranes having a pore size between 1–100 nm, can reject particulates and macromolecules such as proteins, fatty acids, bacteria, protozoa, viruses, and suspended solids. In particular, it can reduce organic matter and BOD (biological oxygen demand) by at least 95%, greatly reduce turbidity, exceed regulatory standards of water quality, achieving 90–100% pathogen removal. Moreover, many municipal water treatment facilities use UF treatment against contamination from cryptosporidium, giardia, and other organisms that could cause serious illness if ingested [29][30][42,43]. Therefore, UF is used to replace existing secondary (sedimentation, flocculation, coagulation) and tertiary filtration (sand filtration and chlorination) methods employed in WWT. In particular, it can permit the reuse of water coming from the industries that consume huge volumes of water or discharge highly toxic effluent such as chemicals, steel, plastics and resins, paper and pulp, pharmaceutical and the food and beverage industries, water and wastewater treatment plants, and etc. [26][39].
UF, despite a broad molecular weight cut off (MWCO) range, is less active in removing low molecular weight organic matters. In many cases, UF is integrated into the process, using primary (flotation and filtration) and some secondary treatments as pretreatment stages and used for pre-filtration in reverse-osmosis plants to protect the reverse-osmosis process.
Today, the ultrafiltration process coupled with the coagulation step is one of the main water treatment technologies in the current water plants proving a significant removal of organic matter in water. However, these technologies are not properly designed for the microplastic removal that remains in the final effluents [22][31][22,44]. Indeed, most of the papers report the removal of the natural organic matter (NOM) that is a complex matrix of organic compounds with a wide variety of chemical properties, chemical composition, and molecular weight [32][33][45,46].
However, the worrying levels of microplastic in freshwaters make a mandatory a-depth investigation of the behavior of microplastic removal during coagulation and ultrafiltration (UF) processes, also considering that they are water treatment technologies used in the production of drinking water [34][35][47,48].
To date, a few papers report the study of microplastic removal by coagulation and UF process for the production of drinking water [36][37][49,50]. In particular, the study reported by Ma et al. 2019 focused on the removal behavior of polyethylene (PE) in drinking water treatment by ultrafiltration and coagulation processes by using an Fe-based coagulant. PE is the most abundant plastic pollutant detected in the water, and moreover, its density (0.92–0.97 g/cm3) very close to that of water makes it difficult to remove by water treatment processes. Low removal efficiency of PE particles (below 15%) was observed after coagulation, indicating the ineffectiveness of the sole coagulation process with respect to microplastic removal. However, when the Polyacrylamide (PAM) was added to enhance the coagulation performance, removal efficiency of small-particle-size PE (d < 0.5 mm) significantly increased from 13 to 91% (Figure 2).
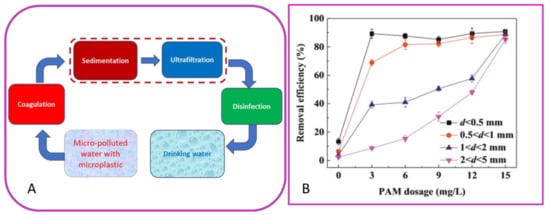

Figure 2. Scheme of the process for removal (A) and removal efficiency (B) of polyethylene (PE) using FeCl3·6H2O and anionic polyacrylamide (PAM)(elaborated from Ma et al. 2019).
For what concerns the UF performance, an interesting result was that the membrane fouling was progressively reduced after coagulation with PE. In particular, by increasing the dosage of coagulant, the porosity of the floc cake layer increased due to the presence of PE particles, especially the large ones. As a result, less severe membrane fouling was induced compared to that with flocs alone. The presence of larger PE particles had positive effects on the membrane fouling. The membrane flux decreased by only 10% in the presence of large-particle-size PE (2 < d < 5 mm) after the coagulation with 0.2 mmol/L PAM and 2 mmol/L FeCl3·6H2O, respectively [37][50]. However, this behavior may not be a general rule but can depend on different parameters related to the membrane process, as well as to the plastic characteristic (chemical composition, size, and shape).
The final comment is that as a general principle, UF could be used to remove PE particles totally, but many efforts are required to understand how the cake layer formation and then the fouling is influenced by the presence of plastic particles. Indeed, also the shape of microplastic can affect their removal in different water treatments;. As reported in Talvitie et al. 2017, a portion of plastic in “fiber shape” is not retained by the WWTPs. Therefore, the final stage treatments have to be properly designed for the removal of the fiber to increase the removal efficiency of plastic.
3. Dynamic Membranes Technology
Recently, DM is emerging as an attractive technology for municipal wastewater treatment [38][39][51,52], surface water treatment [40][53], oily water treatment [41][54], industrial wastewater treatment [42][55], and sludge treatment [43][56].
This technology is based on the formation of a cake layer (dynamic membrane, DM), which acts as a secondary membrane/barrier created when particles and other foulants in the wastewater are filtered through a supporting membrane. Since the filtration mechanism of the DM is quite different compared to the MF/UF processes, in the sense that the fouling and foulants are necessary to create the DM layer, the resistance to filtration is caused exclusively by the layer of the cake. However, thicker layers and dense fouling cause a loss of membrane performance. The parameters that must be taken into consideration to limit the formation of fouling are the same that are involved in the DM formation [44][57].
The DM formation process depends on various parameters relating to the supporting membranes (membrane materials, membrane pore size), to the deposited material (particle size, concentration) and to the operating conditions (operating pressure, cross-flow velocity) [39][52].
DM technology attracted great attention because:
- (i) it employs relatively lower-cost materials compared to traditional membranes (such as mesh, non-woven fabric, and woven filter cloth, and stainless-steel mesh);
- (ii) extra chemicals or other contaminants are not introduced considering that the filtration layer is formed by the contaminants of the influent;
- (iii) the experimental setup is generally more compact than the traditional membrane processes (e.g., for ultrafiltration (UF) and microfiltration (MF)), since DM permeation flux is much larger, the membrane module quantities are saved;
- (iv) the energy supply is lower since DM operates under gravity driving mode, and lower transmembrane pressure is required compared to traditional membranes.
The application of DM technology for micro-plastics removal has been also studied [45][58] because DM is suitable to remove low-density/poorly settling particles. DM technology was applied for micro-particle removal from synthetic wastewater under a gravity-driven operation by using a lab-scale DM filtration setup. The DM was formed on a 90 μm mesh and the synthetic wastewater was prepared with diatomite (AR, Tianjin BASF, D90 = 90.5 μm, meaning that over 90% of the particles in this study are within the defined size range of micro-particles) and tap water. The effluent turbidity was reduced to <1 NTU (Nephelometric Turbidity Unit) after 20 min of filtration, verifying the effective removal of micro-particles by the DM. The transmembrane pressure (TMP) during the DM filtration process (in the range from 80 to 180 mm of water) was lower than that observed for conventional microfiltration and ultrafiltration (16 times lesser than the value obtained for wastewater microfiltration) also reducing the energy consumption. Different influent flux was used (in the range from 9 to 21 L/h), and the linear increase of TMP (at a constant rate) was observed with DM filtration time. At an influent flux of 9 L/h, the effluent turbidity was 4.94 NTU at 10 min and 1.41 NTU at 20 min of filtration time while, at an influent flux of 21 L/h, the effluent turbidity was reduced to 7.14 NTU after 3 min and 1.53 NTU at 5 min of filtration indicating that a higher influent flux facilitated the rapid formation of the DM. The DM formation process was strongly affected by the influent particle concentration. Higher influent particle concentrations resulted in more microparticles being filtered through the supporting mesh, laying the foundation for the rapid formation of the DM layer and a faster effluent turbidity reduction. As a result, increasing flux and influent particle concentration can be used for the control of the DM formation process.
4. Reverse Osmosis
Reverse Osmosis (RO), is actually used in municipal and industrial water treatment systems to purify water using nonporous or nanofiltration membranes (pore size > 2 nm) by removing salts, contaminants, heavy metals, and other impurities. It works by applying a high pressure (10–100 bar) to a concentrated water solution that forces the water through a semipermeable membrane, leaving all the other substances essentially in a more concentrated water solution. It is currently applied also in food and beverage production, biopharmaceutical manufacturing, power generation, production of high purity water, and desalination of brackish waters and seawater, as well in the recovery of industrial and municipal wastewater [46][59].
RO membrane fouling is a major challenge for reliable membrane performance [47][60]. A pretreatment stage is mandatory to maintain the flux rates, to control membrane fouling at industrial scale RO desalination systems, minimizing the membranes cleaning frequency, and prolong the useful life of the RO equipment. Usually, some common pretreatment involves the use of chemicals such as coagulants, antiscalants, oxidizing agents, and disinfectants [47][48][60,61]. Other strategies for fouling mitigation include cleaning, surface modification, and the use of novel membrane materials [48][61]. Nowadays, steady performance in terms of water quality and flux was achieved by the combination of an UF pretreatment with RO in the desalination process [49][62]. A growing trend in the application of combined RO-UF plants for desalination at an industrial scale is reported by Ashfaq et al. 2019. Some plants are reported here: Tuas, Singapore (Capacity: 318,000 m3/day; Year: 2013), Ashdod, Israel (Capacity: 275,000 m3/day; Year: 2013), Ajman, United Arab Emirates (Capacity: 115,000 m3/day; Year: 2012), Tangshan, China (Capacity: 110,000 m3/day; Year: 2012), Teshi, Ghana (Capacity: 60,000 m3/day; Year: 2014), Accra, Ghana (Capacity: 60,000 m3/day; Year: 2014), Red Sea Coast—Saudia Arabia (Capacity: 30,000 m3/day, Year: 2016), Gwangyang, South Korea (Capacity: 30,000 m3/day; Year: 2015) [50][63].
The performance of the RO process with respect to MPs removal was reported by Ziajahromi et al. 2017 [51][31]. They characterized and quantified the microplastic in samples coming from a WWTP that produce a highly treated effluent, including screening and sedimentation, biological treatment, flocculation, disinfection/de-chlorination processes, ultrafiltration, and finally a reverse osmosis (RO) process. Results indicate the presence of microplastic fibers in the samples after RO process. In particular, irregular shaped microplastic were detected and identified by Fourier transform infrared spectroscopy analyses in attenuated total reflectance (ATR-FTIR) as alkyd resin (modified polyester) commonly used in paints. This microplastic detection was attributed by the authors to the occurrence of some membrane defects or simply small openings between pipework, indicating the necessity to ad-hoc design the processes for microplastic removal.
Most of the more performant applications of RO in the microplastic removal are obtained when coupled with membrane bioreactor technology that is hereafter discussed.
5. Membrane Bioreactor (MBR)
Membrane bioreactor (MBR) are systems in which catalysis promoted by biological catalysts (bacteria, enzymes), is coupled to a separation process, operated by a membrane system (generally microfiltration or ultrafiltration) [52][64].
Thanks to the different compartments created by the membrane, a controlled heterogeneous (organic/water)/multiphase (liquid/gas) reaction system can be developed. The different phases can be kept separated (as for example, in a membrane-based solvent extraction process), or they can be dispersed into each other (as in a membrane emulsification process). Besides, the versatility of the technology permits an easy integration with other process (e.g., pervaporation, reverse osmosis) perfectly in line with green chemistry principles, within the logic of process intensification, which offers new and much more opportunities in terms of competitiveness, product quality improvement, process or product novelty and environmental friendliness [53][65].
Nowadays, MBR is deemed as one of the most powerful technologies for efficient municipal and industrial wastewater treatment around the world; however, new emerging fields of application are vastly growing, such as food, pharmaceutical, biorefinery, and biodiesel production [54][66].
6. Polymeric Membranes as Source of Plastic Waste: Recent Advances in Their Reuse and Recycling
Nowadays, membrane technology is widely used in water and wastewater treatment with a well-established market. The world market for Membrane Filtration is expected to grow over the next five years, reaching 7030 million US$ in 2024, from 4710 million US$ in 2019, according to a new GIR (Global Info Research) study [55][78]. The huge spread of membrane processes has raised the need to develop methods to reuse and recycle these materials [56][79]. Many efforts have been made the LIFE+ TRANSFOMEM research project (LIFE13 ENV/ES/000751) [57][80], “Transformation of disposed reverse osmosis membranes into recycled ultra- and nanofiltration membranes”, in which the recycling process of disposed reverse osmosis membranes and their reuse in nanofiltration and ultrafiltration processes have been studied. Project results demonstrated that almost 70% of the membranes are recyclable, and the use of recycled membranes can save between 85% and 95% compared to the acquisition of new commercial membranes. Furthermore, there is a company called “MemRe, RO Membrane Recycling”, based in Germany, which deals with the recycling and reuse of membranes at the end of their life [58][81]. In addition, it should also be noted that the production of membranes is increasingly oriented to the use of new bio-based polymers (recyclable and biodegradable) as an alternative to petrochemical polymers [59][82].
