Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by S. Ganguly.
Magnetic hydrogels (MHGs) are a special class of hydrogel that contain at least one magnetic component in their composition. Generally, MNPs are dispersed in a polymer gel matrix to form MHGs. These hydrogels are special because they are prone to show fluctuations in their physical properties in the presence of an externally applied magnetic field.
- biomedical applications
- magnetic hydrogels
- drug delivery
1. Magnetic Nanoparticles (MNPs) in Hyperthermia
Magnetic nanoparticles (MNPs) are dissipative centers of heat energy obtained from hyperthermia. This was first coined and applied in the year of 1957 [38][1]. Since then, MNP-based hyperthermia research has been on a fast track as MNP-based hyperthermia is quite superior compared to traditional hyperthermia in some aspects [39,40][2][3]. Various types of hyperthermia used by clinicians have been depicted in Figure 21. Traditionally, it can be divided into three areas as adopted by clinicians, such as intestinal [41][4], intraluminal [42][5], and capacitive [43][6]. The advantages are shown also in Figure 21. Tiny MNPs may easily pass through cell walls and, similarly to other nanoparticles, be heated in the presence of an external oscillating magnetic field [44,45,46][7][8][9]. This could cause more sophisticated and precise control of cell heating and necrosis. Sometimes MNPs can be functionalized by some target-specific molecules or surface engineering, which tend to attach to the cell walls [47][10]. This could make much better tissue-specific hyperthermia [48,49][11][12]. When MNPs are utilized, the oscillating magnetic field emits radiation that is only felt by the nanoparticles as opposed to the entire body, which is ideal for non-invasive therapy [50][13]. MNPs can easily permeate through the blood–brain barrier, which is desirable for glioblastoma treatment [51,52][14][15]. MNPs are also deliverable with drug molecules [53][16]. This feature could make MNPs dual-model nanoparticles for serving better therapeutic assays. MNPs are also superior for better dispersion in a target site compared to any bulk implantation. This could maintain the homogeneity of the heating during hyperthermia. Moreover, MNP heating is also effective for further anti-tumoral immunity [54][17]. Improved saturation magnetization of MNPs can also be achieved by synthesis optimization and layer-by-layer growth mechanisms. These characteristics may make them multimodal and therapy-focused nanoparticles [55][18].
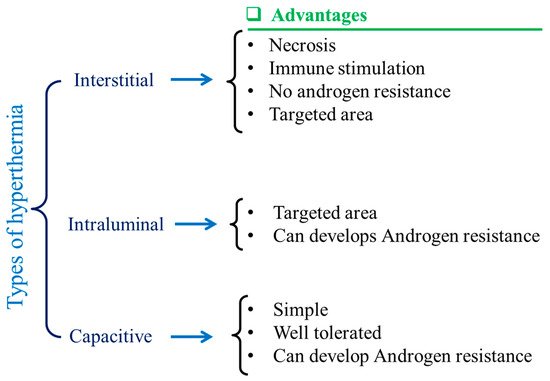
Figure 21. Schematic illustrations of different types of hyperthermia and their advantages.
The most common and widely used MNPs are magnetic (Fe3O4) [56,57][19][20] and maghemite (γ-Fe2O3) [58,59][21][22]. The most promising characteristic of MNPs is their non-cytotoxicity [60][23]. Maghmeite is the oxidized product of magnetite at high temperatures (~300 °C) [61][24]. However, magnetites (MNPs) are more common than maghemite MNPs due to the simplicity of their manufacture and purifying techniques, despite maghemite’s superior thermodynamic stability. As a result, magnetite-based hyperthermia was reported in the majority of MNP-based studies [62][25]. If the MNPs’ size lies within a few nanometers, such as 1–5 nm, cell permeation occurs easily [63][26]. Magnetic behavior is also dependent on the size and shape of MNPs [64][27]. For bulk, magnetic materials’ multi-domain presence is a common thing, but when the size of the material becomes lower the multi-domain particles become single-domain [56,65][19][28]. By this approach, multi-domain materials turn from ferromagnets into superparamagnets [66][29].
The hyperthermia mechanism can be classified into two broad segments; one is hysteresis loss and another one is Néel relaxation loss [67,68][30][31]. There is one aspect that these two systems have in common: they are not reliant on the optimal particle size. In terms of hysteresis loss behavior, multi-domain ferromagnets are inferior to single-domain ferromagnets. When compared to multi-domain ferromagnets, single-domain ferromagnets emit a substantial amount of heat. Hysteresis loss is not present in superparamagnetic nanoparticles. Superparamagnetic nanoparticles generate heat in the presence of alternating magnetic fields because of the relaxation loss phenomenon, especially Néel relaxation loss.
2. Hyperthermia-Based Cancer Treatment
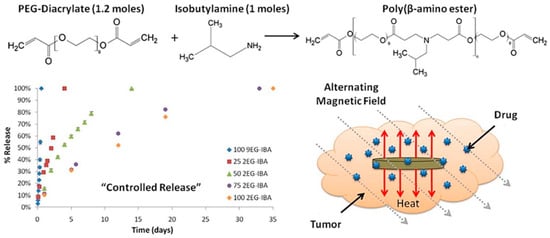
Hyperthermia treatment is performed at 41–46 °C alongside chemotherapy or irradiation to achieve better results in pancreatic cancer and glioblastoma [120,121,122][32][33][34]. However, the precise control of tissue temperature is still a difficult task for clinicians [121,123,124][33][35][36]. For several decades hyperthermia has been used as a radiosensitizer and chemosensitizer, resulting in significant improvements in cancer diagnosis and treatment. This combined hyperthermia technique has been demonstrated to be highly successful in the treatment of malignancies such as bladder cancer, cervical cancer, breast cancer, head–neck cancer, melanoma, and soft-tissue cancers. The mechanism of hyperthermia can be explained as the delivery of heat to the affected region, but it can be performed in various ways. Hyperthermia directly affects the cellular components and delays lethal activity towards cellular responses. DNA repair pathways and a good systemic immune response are among the activities observed in cells following heat treatment. Furthermore, heat affects hypoxic and nutrient-depleted tumor regions, whereas radiation and chemotherapy do not require such monitoring. Besides these, hyperthermia also affects tumor growth, oxygen supply pathways, and vascularization. However, there are three important considerations to keep in mind when using hyperthermia in clinical systems, as indicated by clinicians: First, the temperature rise should be exact and focused. The second step is to regulate the temperature in the affected area, rather than in other parts of the tumor. The last one is the optimization of heat/dose of the hyperthermia, as per the condition of the patient’s body. Hyperthermia systems are quite wide depending on the applied frequency ranges: they are classified as radiofrequency (RF), ultrasound, infrared (IR), and microwave (>300 MHz). The formation of eddy currents (for high electrically conducting samples), magnetization reversal (for magnetic materials), and dipolar motions of magnetic dipoles might all be part of the hyperthermia mechanism. Eddy current production is an outcome of low induction and is not restricted to magnetic materials. It is often used for a wide range of macroscopic materials with high electrical conductivity. When an electrically conducting material is subjected to an alternating material field, an eddy current is created (AMF). A Brownian connection is used for magnetic dipolar movement, resulting in heat generation. The system for MGHs, on the other hand, is a composite in which MNPs are detained but the polymer chains are not. Polymer macrochains are physisorped on the surfaces of MNPs, followed by the full restriction of rotation and movement. As a result, the Brownian relaxation process is not one of the established mechanistic paths proposed by researchers. Néel relaxation is the adopted hypothetical way to explain the heat generation inside MGHs. MHGs provide better results in this context because of their tissue mimetic behavior and remote control of intrinsic features [125,126][37][38]. As previously reported, a PVA-based magnetite-MNP-loaded composite hydrogel demonstrated a rapid temperature rise [127][39]. When a 357 kHz alternating magnetic field was applied, the temperature rose from 43 °C to 47 °C in 5–6 min. From the result it was also inferred that the heating efficiency was directly related to the MNPs present in the system. Similarly, in another work Fe3O4 microparticles were used to prepare PNIPAM-based thermoresponsive hydrogels [128][40]. The specific adsorption rate (SAR) is an important measure for hyperthermia researchers. The quantity of heat emitted by a substance in a given amount of time is known as the SAR. It is also dependent on the external magnetic field strength. It is mathematically defined as c(ΔT/Δt), where ‘c’ and ‘ΔT/Δt’ correspond to the specific heat capacity and time-dependent temperature increment, respectively. It is critical to increase or improve the SAR value by as much as is feasible. The SAR is affected by a number of elements, including the intensity of the external magnetic field, the frequency of the alternating current, the permeability of the particles under test (in this case, MNPs), and the shape and size distribution of the MNPs. Anderson et al. fabricated PEG-based MHGs which showed temperature rising at the hyperthermia range as well as in the thermoablation range (61–64 °C) [129][41]. In their work they showed that cell necrosis was observed against gliobastoma cells. The same group also reported a poly(β-amino ester)-based biodegradable hydrogel (Figure 72) for hyperthermia treatment where the hydrogel was remotely controlled by an external magnetic field [130][42].
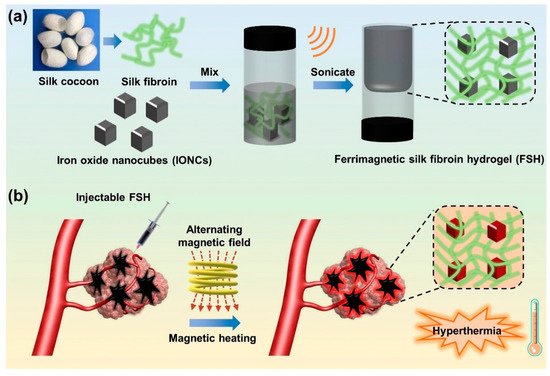
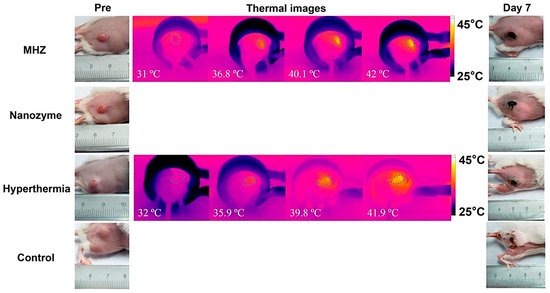
Besides hyperthermia-based drug delivery, MNP-based hydrogels are also used as a targeted tumor treatment. The tumor microenvironment has a critical microstructure with uncommon biological features, such as acidosis and high glutathione content, compared to normal cells. Wu et al. reported a magnetic, injectable hydrogel for tumor treatment by hyperthermia [131][43]. They used PEGylated MNPs and cyclodextrin to prepare a nanoenzyme hydrogel which showed temperature increments of up to 42 °C. Injectable hydrogels are superior compared to traditional macroscopic hydrogels due to target-specific activity and easy reach to the infected area. Combinational therapy was also reported in this work, showing synergy between drug release and hyperthermia. They showed that the synergy of drug release and hyperthermia cured a tumor within 7 days of treatment in several intervals. The treatment was monitored by an infrared camera to evaluate the exact position of heating, as shown in Figure 83.
Chen et al., fabricated a ferumoxytol-medical-chitosan-based hydrogel which showed tumor apoptosis in the presence of an alternating magnetic field [132][44]. Furthermore, they demonstrated that when an anti-cancer agent (in this case, doxorubicin) is coupled to the MNP-based hydrogel it improves xenograft tumor treatment effectiveness. Sol–gel transitions in hydrogel systems are another method for delivering molecular payloads and implantations to specified areas of the body without invasive paths. In this case, injectable hydrogels are appropriate since they gel quickly at body temperature. Injectable hydrogels are beneficial in the treatment of localized hyperthermia. Thermoresponsive polymers, which have been used to fabricate MHGs, are quite common in this situation. There are several limitations however, such as the adjustment and optimization of MNP concentration, viscosity of the hydrogel after the incorporation of MNPs, and undesired migration of MNPs beside the targeted site. Gelatin-based MHGs were reported for the synergistic application of hyperthermia and chemotherapy [95][45]. Methacrylic-anhydride-functionalized gelatin was copolymerized with 2-dimethylaminoethyl) methacrylate, with methacrylate-end-capped magnetic nanoparticles serving as the MNPs. This hydrogel has built-in magnetism and pH sensitivity, making it a dual-responsive gadget. Salloum et al. fabricated a ferrofluid-based injectable agarose gel for hyperthermia applications [133][46]. Qian et al. prepared a PEG-stabilized iron-oxide-nanocube-loaded silk fibroin hydrogel for antitumor therapy [134][47]. This hydrogel (Figure 94) showed shear thinning behavior and was applied as an injectable hydrogel. The prepared hydrogel was injected into a rabbit liver tumor and heated with an external oscillating magnetic field followed by thermoablation of the cells. Another important property of the hydrogel is its injectability, which allows for the rapid delivery of molecular payloads into specified areas via a less invasive manner. The law of sol–gel flow behavior applies to injectable hydrogels. A distinct type of hydrogel, in which substantial intermolecular interactions predominate in gel matrices, has demonstrated a solution to gelation. The physical cross-linking of these hydrogels is takes place (H-bonding, hydrophobic association, and van der Waals interactions). Jordan et al. reported injectable hydrogels based on chitosan and a block copolymer (poloxamer 407). Block copolymers show excellent sol–gel transitions with an alteration in temperature. Biopolymers and superparamagnetic iron oxide nanoparticles were employed as an additional phase in these hydrogels (SPIONs) [135][48]. SPIONs of 20% (w/v) were incorporated into thermoresponsive polymer matrices and injected for implantation, followed by heating with an AMF. Similarly, a 10% (w/v)-SPION-loaded biopolymer hydrogel was prepared by the ionic gelation method and injected into tumor sites. Among the block-copolymer-based injectable hydrogels, poly(ethylene-co-vinyl alcohol) (EVAL) is a significant name. An EVAL-based SPION-loaded hydrogel was reported which acted as an injectable hydrogel and showed a high SAR value when heated by an AMF [136][49]. Rheology is commonly used to determine injectability. SPIONs with a large surface area are prone to adsorption by polymers, limiting the flow behavior of composites. SPIONs additionally improve the thixotropic character of the material and postpone network rupturing during shear stress. When injectable hydrogels are pressed to be inserted into the body by a fine diameter nozzle, they suffer from high shear stress. The SPIONs offer the gel the strength required to hold the composite in place during the procedure without premature rupture. In SPION-based injectable hydrogels, gelation at body temperature and insolubility are also non-negotiable characteristics. In general, in specific polymer concentrations, pluronic-type hydrogels display a good transition from a solution into a gel phase. Pluronics are block copolymers that dissolve in water at room temperature. However, at a certain concentration they gel at a specified temperature, which are referred to as the critical gel concentration and critical gelling temperature. As strength is the primary quality of any injectable, filler particles are introduced. SPIONs act as a reinforcement in the hydrogel and also maintain their dimensional integrity inside the body. Moreover, the heating capability of such hydrogels is also not compromised. For injectable MHGs, the target and press ion are much more accurate than the macroscopic hydrogels of bulks. These MHGs can be injected into the exact location and easily heated by an AMF.
3. Applications in Drug Delivery
Hydrogels are soft materials arranged in three dimensions by hydrophilic polymeric networks [137][50]. Hydrogels have several unique physical features due to their porous structure, making them a good material for drug couriers and controlled release of drug molecules under specified environmental conditions [138,139][51][52]. The release of drug molecules from a hydrogel matrix is dependent on their diffusion behavior [140,141][53][54]. Drug molecules are imbibed into the hydrogel network and captured by the hydrogel matrix when polymeric hydrogels are submerged in a drug solution [142][55]. During some special environmental conditions the drug molecules come out from the hydrogel matrix by obeying Fick’s diffusion law or some anomalous diffusion kinetics [143][56]. Such behavior is also shown by different nano-drug carriers [144,145][57][58]. In the presence of some external stimuli, most hydrogels are particularly vulnerable to having their internal microstructure and porosity manipulated [146][59]. The most common stimuli for hydrogel systems are the pH of a solution, an electric field, temperature, a saline environment, light, enzymes, and a magnetic field [147,148][60][61]. Among the external stimuli, the magnetic field is comparatively new with respect to the others. The magnetic field might be constant or variable in frequency. A frequency-dependent magnetic field, also known as an alternating magnetic field or an oscillating magnetic field, is one in which the magnetic dipoles are activated, causing the magnetic nanoparticles to be heated.
Gelatin and magnetic nanoparticles were combined together to prepare a magnetic composite hydrogel where genipin was used as a cross-linking agent [149][62]. Vitamin B12 was utilized as a model payload in this hydrogel system, and it diffused out of the hydrogel matrix in the presence of an external magnetic field. The amount of vitamin B12 released from the hydrogel matrix was proportional to the duration of the magnetic field. This suggests that MHGs might be regulated by external magnetic fields, which could govern the pace of release and dosage. In another work, chitosan and sodium alginate were taken to fabricate MHGs regulated by an external magnetic field [150][63]. The author employed a responsive polymer in this ferrogel to induce temperature-dependent medication release. Another set of researchers reported carboxymethyl cellulose (CMC)- and iron-oxide-based nanocomposite MHGs [151][64]. In this work they also showed how external magnetic fields can influence the cumulative release percentage of any model drug. A 2-hydroxyethyl methacrylate and iron oxide composite hydrogel has previously been reported to prepare microrobots to deliver anti-cancer drugs to a specific section. Huang et al. described an MNP-based copolymer hydrogel with triple-responsive behavior to pH, temperature, and glucose [152][65]. These hydrogels showed self-regulatory drug release and superparamagnetic behavior. Tragacanth gum (TG)- and poly(acrylic acid)-based MHGs were reported to prepare a smart drug delivery system [153][66]. Here, the MNPs were magnetite and the hydrogel showed cell apoptosis against a HeLa cell line. Cao et al. developed double-network MHGs from polyacrylamide and alginate [154][67]. The hydrogel was tough and compressible (Figure 105). This was used in magnetic robots in underwater applications as well as an efficient drug delivery device. They proposed a straightforward method for making magnetic hydrogels with good mechanical properties by combining physical mixing and chemical cross-linking procedures in their work. MNPs were fine-tuned and a fast magnetic response was created. The magnetic hydrogel was utilized to make two standard magnetically responsive marine animal robots (a scallop and a starfish) that were used to clean the fish tank using a remotely controlled magnet. The suggested technique may be applied to different hydrogel systems, expanding the range of smart hydrogel applications.
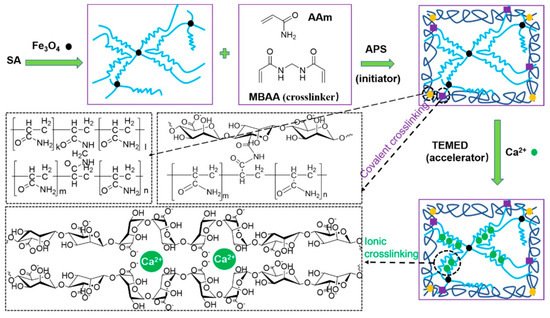
Figure 105. Schematic of reaction pathway to prepare a sodium alginate (SA)-based double-network magnetic hydrogel. During the gelation of ammonium persulfate (APS) N,N,N′,N′-tetramethylethylenediamine (TEMED) and N,N′-methylenebis(acrylamide) (MBAA) were used as an activator and cross-linker, respectively [154][67] © 2021 American Chemical Society.
In another work, a nanocomposite hydrogel was prepared from the dopamine–Fe3+ complex and reinforced with MNPs [155][68]. The authors discovered that MNPs had a significant impact on their shear modulus. They tried a combinational approach for the cancer treatment. They mingled the hyperthermia and targeted drug delivery into one system and showed better efficacy towards cancer treatment. The hydrogel was regulated by the external magnetic field and could be heated in a non-contact mode. When an external AMF was turned on, the nanocomposite hydrogel showed a pulsed release of an anti-cancer drug (DOX), but when the AMF was turned off it reverted to its slow releasing mode. In addition, in vivo, the DOX-loaded composite hydrogel had a longer retention duration than the DOX-loaded gel or DOX solution. They also hypothesized whether if a single-modal treatment was carried out, i.e., only with an anti-cancer drug or hyperthermia, the curing would be as effective as the combined synergy (Figure 116). The live–dead assay of the cell line also implied that magnetic fields and anti-cancer drugs together can cause cell death easier than using a single tool.
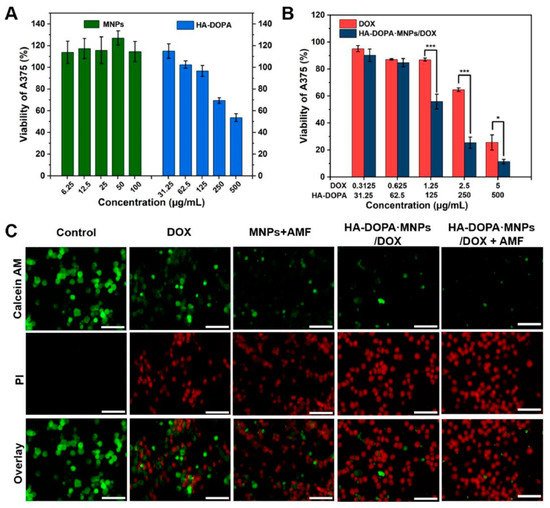
Figure 116. (A) Viabilities of human melanoma cells (A375) after 24 h of treatment with MNPs and an injectable hydrogel made of dopamine-conjugated hyaluronan (HA-DOPA). (B) Viabilities of A375 cells after 24 h of treatment with an anti-cancer drug (doxorubicin; DOX) and an anti-cancer-drug-loaded hydrogel. (C) Fluorescence images of A375 cells with staining of calcein AM (AM, green, live cells) and propidium iodide (PI, red, dead cells) after various treatments. The images show how magnetic fields and anti-cancer drugs both act on cell death. Scale bar: 100 µm [155][68].
References
- Selawry, O.S.; Goldstein, M.N.; McCormick, T. Hyperthermia in tissue-cultured cells of malignant origin. Cancer Res. 1957, 17, 785–791.
- García-Hevia, L.; Casafont, Í.; Oliveira, J.; Terán, N.; Fanarraga, M.L.; Gallo, J.; Bañobre-López, M. Magnetic lipid nanovehicles synergize the controlled thermal release of chemotherapeutics with magnetic ablation while enabling non-invasive monitoring by MRI for melanoma theranostics. Bioact. Mater. 2022, 8, 153–164.
- Datta, N.R.; Kok, H.P.; Crezee, H.; Gaipl, U.S.; Bodis, S. Integrating loco-regional hyperthermia into the current oncology practice: SWOT and TOWS analyses. Front. Oncol. 2020, 10, 819.
- Walter, E.; Watt, P.W.; Gibson, O.R.; Wilmott, A.G.; Mitchell, D.; Moreton, R.; Maxwell, N.S. Exercise hyperthermia induces greater changes in gastrointestinal permeability than equivalent passive hyperthermia. Physiol. Rep. 2021, 9, e14945.
- Bachmann, C.; Sautkin, I.; Nadiradze, G.; Archid, R.; Weinreich, F.; Königsrainer, A.; Reymond, M. Technology development of hyperthermic pressurized intraperitoneal aerosol chemotherapy (hPIPAC). Surg. Endosc. 2021, 35, 6358–6365.
- Szasz, A. The Capacitive Coupling Modalities for Oncological Hyperthermia. Open J. Biophys. 2021, 11, 252–313.
- Das, P.; Ganguly, S.; Saha, A.; Noked, M.; Margel, S.; Gedanken, A. Carbon-dots-initiated photopolymerization: An in situ synthetic approach for MXene/poly (norepinephrine)/copper hybrid and its application for mitigating water pollution. ACS Appl. Mater. Interfaces 2021, 13, 31038–31050.
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031.
- Das, P.; Ganguly, S.; Mondal, S.; Ghorai, U.K.; Maity, P.P.; Choudhary, S.; Gangopadhyay, S.; Dhara, S.; Banerjee, S.; Das, N.C. Dual doped biocompatible multicolor luminescent carbon dots for bio labeling, UV-active marker and fluorescent polymer composite. Luminescence 2018, 33, 1136–1145.
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; Johannsen, M.; Wust, P.; Nadobny, J.; Schirra, H.; Schmidt, H.; Deger, S.; Loening, S. Presentation of a new magnetic field therapy system for the treatment of human solid tumors with magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2001, 225, 118–126.
- Gavazzi, S.; van Lier, A.L.; Zachiu, C.; Jansen, E.; Lagendijk, J.J.W.; Stalpers, L.J.A.; Crezee, H.; Kok, H.P. Advanced patient-specific hyperthermia treatment planning. Int. J. Hyperth. 2020, 37, 992–1007.
- Suleman, M.; Riaz, S. In silico study of enhanced permeation and retention effect and hyperthermia of porous tumor. Med. Eng. Phys. 2020, 86, 128–137.
- Molcan, M.; Kaczmarek, K.; Kubovcikova, M.; Gojzewski, H.; Kovac, J.; Timko, M.; Józefczak, A. Magnetic hyperthermia study of magnetosome chain systems in tissue-mimicking phantom. J. Mol. Liq. 2020, 320, 114470.
- Wolburg, H.; Noell, S.; Fallier-Becker, P.; Mack, A.F.; Wolburg-Buchholz, K. The disturbed blood–brain barrier in human glioblastoma. Mol. Asp. Med. 2012, 33, 579–589.
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021.
- Varmazyar, M.; Habibi, M.; Amini, M.; Pordanjani, A.H.; Afrand, M.; Vahedi, S.M. Numerical simulation of magnetic nanoparticle-based drug delivery in presence of atherosclerotic plaques and under the effects of magnetic field. Powder Technol. 2020, 366, 164–174.
- Schürch, C.M.; Bhate, S.S.; Barlow, G.L.; Phillips, D.J.; Noti, L.; Zlobec, I.; Chu, P.; Black, S.; Demeter, J.; McIlwain, D.R. Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell 2020, 182, 1341–1359.e1319.
- Kuhn, J.; Papanastasiou, G.; Tai, C.-W.; Moran, C.M.; Jansen, M.A.; Tavares, A.A.; Lennen, R.J.; Corral, C.A.; Wang, C.; Thomson, A.J. Tri-modal imaging of gold-dotted magnetic nanoparticles for magnetic resonance imaging, computed tomography and intravascular ultrasound: An in vitro study. Nanomedicine 2020, 15, 2433–2445.
- Amara, D.; Felner, I.; Nowik, I.; Margel, S. Synthesis and characterization of Fe and Fe3O4 nanoparticles by thermal decomposition of triiron dodecacarbonyl. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 106–110.
- Gordon, T.; Perlstein, B.; Houbara, O.; Felner, I.; Banin, E.; Margel, S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 1–8.
- Ganguly, S.; Margel, S. Remotely controlled magneto-regulation of therapeutics from magnetoelastic gel matrices. Biotechnol. Adv. 2020, 44, 107611.
- Molinari, S.; Magro, M.; Baratella, D.; Salviulo, G.; Ugolotti, J.; Filip, J.; Petr, M.; Tucek, J.; Zoppellaro, G.; Zboril, R. Smart synthetic maghemite nanoparticles with unique surface properties encode binding specificity toward AsIII. Sci. Total. Environ. 2020, 741, 140175.
- Ziv-Polat, O.; Topaz, M.; Brosh, T.; Margel, S. Enhancement of incisional wound healing by thrombin conjugated iron oxide nanoparticles. Biomaterials 2010, 31, 741–747.
- Ganguly, S.; Grinberg, I.; Margel, S. Layer by layer controlled synthesis at room temperature of tri-modal (MRI, fluorescence and CT) core/shell superparamagnetic IO/human serum albumin nanoparticles for diagnostic applications. Polym. Adv. Technol. 2021, 32, 3909–3921.
- Ziv, O.; Avtalion, R.R.; Margel, S. Immunogenicity of bioactive magnetic nanoparticles: Natural and acquired antibodies. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 85, 1011–1021.
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron oxide nanoparticles for neuronal cell applications: Uptake study and magnetic manipulations. J. Nanobiotechnol. 2016, 14, 37.
- Ziv-Polat, O.; Skaat, H.; Shahar, A.; Margel, S. Novel magnetic fibrin hydrogel scaffolds containing thrombin and growth factors conjugated iron oxide nanoparticles for tissue engineering. Int. J. Nanomed. 2012, 7, 1259.
- Skaat, H.; Margel, S. Synthesis of fluorescent-maghemite nanoparticles as multimodal imaging agents for amyloid-β fibrils detection and removal by a magnetic field. Biochem. Biophys. Res. Commun. 2009, 386, 645–649.
- Zhang, H.; Moore, L.R.; Zborowski, M.; Williams, P.S.; Margel, S.; Chalmers, J.J. Establishment and implications of a characterization method for magnetic nanoparticle using cell tracking velocimetry and magnetic susceptibility modified solutions. Analyst 2005, 130, 514–527.
- Ganguly, S.; Kanovsky, N.; Das, P.; Gedanken, A.; Margel, S. Photopolymerized Thin Coating of Polypyrrole/Graphene Nanofiber/Iron Oxide onto Nonpolar Plastic for Flexible Electromagnetic Radiation Shielding, Strain Sensing, and Non-Contact Heating Applications. Adv. Mater. Interfaces 2021, 2101255.
- Skaat, H.; Sorci, M.; Belfort, G.; Margel, S. Effect of maghemite nanoparticles on insulin amyloid fibril formation: Selective labeling, kinetics, and fibril removal by a magnetic field. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 91, 342–351.
- Rego, G.N.; Nucci, M.P.; Mamani, J.B.; Oliveira, F.A.; Marti, L.C.; Filgueiras, I.S.; Ferreira, J.M.; Real, C.C.; de Paula Faria, D.; Espinha, P.L.; et al. Therapeutic efficiency of multiple applications of magnetic hyperthermia technique in glioblastoma using aminosilane coated iron oxide nanoparticles: In vitro and in vivo study. Int. J. Mol. Sci. 2020, 21, 958.
- Gupta, R.; Tomar, R.; Chakraverty, S.; Sharma, D. Effect of manganese doping on the hyperthermic profile of ferrite nanoparticles using response surface methodology. RSC Adv. 2021, 11, 16942–16954.
- Dhiman, N.; Shagaghi, N.; Bhave, M.; Sumer, H.; Kingshott, P.; Rath, S.N. Indirect co-culture of lung carcinoma cells with hyperthermia-treated mesenchymal stem cells influences tumor spheroid growth in a collagen-based 3-dimensional microfluidic model. Cytotherapy 2021, 23, 25–36.
- Senturk, F.; Cakmak, S.; Kocum, I.C.; Gumusderelioglu, M.; Ozturk, G.G. GRGDS-conjugated and curcumin-loaded magnetic polymeric nanoparticles for the hyperthermia treatment of glioblastoma cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126648.
- Chandunika, R.; Vijayaraghavan, R.; Sahu, N.K. Magnetic hyperthermia application of MnFe2O4 nanostructures processed through solvents with the varying boiling point. Mater. Res. Express 2020, 7, 064002.
- Gupta, R.; Sharma, D. (Carboxymethyl-stevioside)-coated magnetic dots for enhanced magnetic hyperthermia and improved glioblastoma treatment. Colloids Surf. B Biointerfaces 2021, 205, 111870.
- Frenster, J.D.; Desai, S.; Placantonakis, D.G. In vitro evidence for glioblastoma cell death in temperatures found in the penumbra of laser-ablated tumors. Int. J. Hyperth. 2020, 37, 20–26.
- Lao, L.; Ramanujan, R. Magnetic and hydrogel composite materials for hyperthermia applications. J. Mater. Sci. Mater. Med. 2004, 15, 1061–1064.
- Ang, K.; Venkatraman, S.; Ramanujan, R. Magnetic PNIPA hydrogels for hyperthermia applications in cancer therapy. Mater. Sci. Eng. C 2007, 27, 347–351.
- Meenach, S.A.; Hilt, J.Z.; Anderson, K.W. Poly (ethylene glycol)-based magnetic hydrogel nanocomposites for hyperthermia cancer therapy. Acta Biomater. 2010, 6, 1039–1046.
- Meenach, S.A.; Otu, C.G.; Anderson, K.W.; Hilt, J.Z. Controlled synergistic delivery of paclitaxel and heat from poly (β-amino ester)/iron oxide-based hydrogel nanocomposites. Int. J. Pharm. 2012, 427, 177–184.
- Wu, H.; Liu, L.; Song, L.; Ma, M.; Gu, N.; Zhang, Y. Enhanced tumor synergistic therapy by injectable magnetic hydrogel mediated generation of hyperthermia and highly toxic reactive oxygen species. ACS Nano 2019, 13, 14013–14023.
- Chen, B.; Xing, J.; Li, M.; Liu, Y.; Ji, M. Ferumoxytol-Medical Chitosan as magnetic hydrogel therapeutic system for effective magnetic hyperthermia and chemotherapy in vitro. Colloids Surf. B Biointerfaces 2020, 190, 110896.
- Derakhshankhah, H.; Jahanban-Esfahlan, R.; Vandghanooni, S.; Akbari-Nakhjavani, S.; Massoumi, B.; Haghshenas, B.; Rezaei, A.; Farnudiyan-Habibi, A.; Samadian, H.; Jaymand, M. A bio-inspired gelatin-based pH-and thermal-sensitive magnetic hydrogel for in vitro chemo/hyperthermia treatment of breast cancer cells. J. Appl. Polym. Sci. 2021, 138, 50578.
- Salloum, M.; Ma, R.; Weeks, D.; Zhu, L. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: Experimental study in agarose gel. Int. J. Hyperth. 2008, 24, 337–345.
- Qian, K.-Y.; Song, Y.; Yan, X.; Dong, L.; Xue, J.; Xu, Y.; Wang, B.; Cao, B.; Hou, Q.; Peng, W. Injectable ferrimagnetic silk fibroin hydrogel for magnetic hyperthermia ablation of deep tumor. Biomaterials 2020, 259, 120299.
- Le Renard, P.-E.; Jordan, O.; Faes, A.; Petri-Fink, A.; Hofmann, H.; Ruefenacht, D.; Bosman, F.; Buchegger, F.; Doelker, E. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterials 2010, 31, 691–705.
- Le Renard, P.-E.; Lortz, R.; Senatore, C.; Rapin, J.-P.; Buchegger, F.; Petri-Fink, A.; Hofmann, H.; Doelker, E.; Jordan, O. Magnetic and in vitro heating properties of implants formed in situ from injectable formulations and containing superparamagnetic iron oxide nanoparticles (SPIONs) embedded in silica microparticles for magnetically induced local hyperthermia. J. Magn. Magn. Mater. 2011, 323, 1054–1063.
- Ganguly, S.; Maity, T.; Mondal, S.; Das, P.; Das, N.C. Starch functionalized biodegradable semi-IPN as a pH-tunable controlled release platform for memantine. Int. J. Biol. Macromol. 2017, 95, 185–198.
- Doktorovova, S.; Souto, E.B. Nanostructured lipid carrier-based hydrogel formulations for drug delivery: A comprehensive review. Expert Opin. Drug Deliv. 2009, 6, 165–176.
- Ganguly, S.; Das, N.C. Synthesis of Mussel Inspired Polydopamine Coated Halloysite Nanotubes Based Semi-IPN: An Approach to Fine Tuning in Drug Release and Mechanical Toughening. In Proceedings of the Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2018; p. 1800076.
- Ganguly, S.; Maity, P.P.; Mondal, S.; Das, P.; Bhawal, P.; Dhara, S.; Das, N.C. Polysaccharide and poly (methacrylic acid) based biodegradable elastomeric biocompatible semi-IPN hydrogel for controlled drug delivery. Mater. Sci. Eng. C 2018, 92, 34–51.
- Ganguly, S. Preparation/processing of polymer-graphene composites by different techniques. In Polymer Nanocomposites Containing Graphene; Elsevier: New York, NY, USA, 2022; pp. 45–74.
- Falk, B.; Garramone, S.; Shivkumar, S. Diffusion coefficient of paracetamol in a chitosan hydrogel. Mater. Lett. 2004, 58, 3261–3265.
- Lee, P.I. Kinetics of drug release from hydrogel matrices. J. Control. Release 1985, 2, 277–288.
- Das, P.; Ganguly, S.; Agarwal, T.; Maity, P.; Ghosh, S.; Choudhary, S.; Gangopadhyay, S.; Maiti, T.K.; Dhara, S.; Banerjee, S. Heteroatom doped blue luminescent carbon dots as a nano-probe for targeted cell labeling and anticancer drug delivery vehicle. Mater. Chem. Phys. 2019, 237, 121860.
- Ganguly, S.; Das, P.; Das, S.; Ghorai, U.; Bose, M.; Ghosh, S.; Mondal, M.; Das, A.K.; Banerjee, S.; Das, N.C. Microwave assisted green synthesis of Zwitterionic photolumenescent N-doped carbon dots: An efficient ‘on-off’chemosensor for tracer Cr (+6) considering the inner filter effect and nano drug-delivery vector. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123604.
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019, 98, 101147.
- Prabaharan, M.; Mano, J.F. Stimuli-responsive hydrogels based on polysaccharides incorporated with thermo-responsive polymers as novel biomaterials. Macromol. Biosci. 2006, 6, 991–1008.
- Ganguly, S.; Das, N.C. Water uptake kinetics and control release of agrochemical fertilizers from nanoclay-assisted semi-interpenetrating sodium acrylate-based hydrogel. Polym.-Plast. Technol. Eng. 2017, 56, 744–761.
- Liu, T.-Y.; Hu, S.-H.; Liu, K.-H.; Liu, D.-M.; Chen, S.-Y. Preparation and characterization of smart magnetic hydrogels and its use for drug release. J. Magn. Magn. Mater. 2006, 304, e397–e399.
- Hernández, R.; Sacristán, J.; Asín, L.; Torres, T.; Ibarra, M.; Goya, G.; Mijangos, C. Magnetic hydrogels derived from polysaccharides with improved specific power absorption: Potential devices for remotely triggered drug delivery. J. Phys. Chem. B 2010, 114, 12002–12007.
- Reddy, N.N.; Mohan, Y.M.; Varaprasad, K.; Ravindra, S.; Joy, P.; Raju, K.M. Magnetic and electric responsive hydrogel–magnetic nanocomposites for drug-delivery application. J. Appl. Polym. Sci. 2011, 122, 1364–1375.
- Huang, Y.; Liu, M.; Chen, J.; Gao, C.; Gong, Q. A novel magnetic triple-responsive composite semi-IPN hydrogels for targeted and controlled drug delivery. Eur. Polym. J. 2012, 48, 1734–1744.
- Sayadnia, S.; Arkan, E.; Jahanban-Esfahlan, R.; Sayadnia, S.; Jaymand, M. Tragacanth gum-based pH-responsive magnetic hydrogels for “smart” chemo/hyperthermia therapy of solid tumors. Polym. Adv. Technol. 2021, 32, 262–271.
- Cao, Q.; Liu, N.; Xiao, Y.; Huang, R.; Li, Y.; Wu, L. Hybrid Magnetic Hydrogels Used as Artificial Marine Animals for Noncontact Cleaning. ACS Appl. Polym. Mater. 2021, 3, 1182–1189.
- Dai, G.; Sun, L.; Xu, J.; Zhao, G.; Tan, Z.; Wang, C.; Sun, X.; Xu, K.; Zhong, W. Catechol–metal coordination-mediated nanocomposite hydrogels for on-demand drug delivery and efficacious combination therapy. Acta Biomater. 2021, 21, 958.
More