1. Background
Fat is one of the essential components of food products, playing a lot of critical technological functions. It affects its sensory characteristics, such as taste and aroma, and textural and physical properties
[1][2][3][1,2,3]. The food industry most commonly uses naturally solid or modified liquid fats, which contain unsaturated or saturated fatty acids of trans configuration
[4]. Fatty acid isomers of trans configuration have a beneficial effect on the texture of fats, but when consumed in higher amounts, they contribute to cardiovascular disease and metabolic disorders
[5].
Different methods are used in food production to convert liquid oils into solid fats, like hydrogenation, fractionation, or interesterification
[2]. The most commonly used method is the hydrogenation of unsaturated fatty acids of vegetable oils, which produces saturated solid fats characterized by improved oxidative stability and plasticity. This process, although technologically advantageous, carries the risk of creating the trans fatty acids negatively impacting on human health.
Interestrification, which significantly reduces the content of trans fatty acids in modified fats, cannot fully eliminate them, translating into health problems for consumers
[6]. Conversely, removing the saturated acids would result in abnormal physical and sensory changes in typical product that were unacceptable to consumers
[2][7][2,7].
An alternative solution to the previously mentioned problems is the concept of oleogels in food production. Oleogelation may become a promising method in the future for curing liquid fats rich in unsaturated fatty acids. This process can change the consistency of liquid vegetable or fish oils and give them the properties of solid fats, without the participation and presence of harmful saturated trans fatty acids in their composition.
The oleogelation method involves creating a semi-solid oil form using gelling substances
[8]. Oil structuring is based on the physical transformation of dissolved gelators in a lipid environment whose chemical characteristics are constant during the process
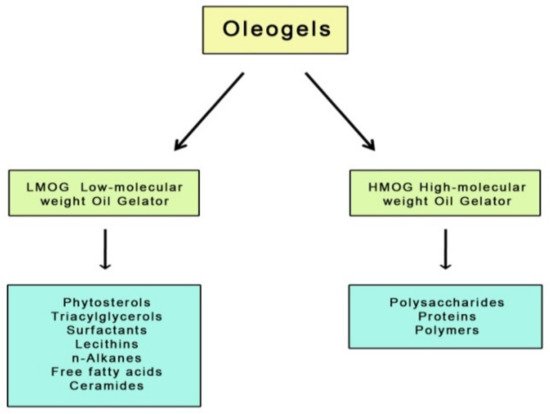
[9]. Gelators facilitating solid structure development can be divided into two groups; these are low molecular weight oil gelators (LMOG) and high molecular weight oil gelators (HMOG) (
Figure 1).
Figure 1. Gelator compounds of different molecular weight.
The first group refers to small molecules that build a fixed crystalline network stabilizing the oil phase through self-organization. This process occurs under controlled temperature conditions and is driven by existing physical interactions between the molecules, such as hydrogen bonds, hydrophobic bonds, and Van der Waals interactions. Due to the formation mechanism and physical interactions, the structure of these oleogels is susceptible to shear forces and temperature
[10].
LMOGs include, for example, naturally occurring simple sugars (e.g., trehalose, sucrose, mannose, amygdaline), glycosides (raspberry glycoside ketone, salicin), and sugar alcohols (e.g., xylitol, sorbitol, mannitol)
[11], but also waxes, fatty acids, carbamates, lecithins, ceramides, monoacylglycerols, diacylglycerols, triacylglycerols, n-alkanes, and mixtures of γ-oryzanol and phytosterols
[12][13][12,13].
The second group of gelators (HMOGs) includes macromolecular systems capable of forming three-dimensional networks linked to each other by physical interactions such as hydrogen bonds. Considering the polymeric properties of these networks, oleogels built with such gelators have better viscoelastic properties, which are determined by the concentration, molecular weight, and conformation of the polymer
[10]. High molecular weight gelling agents used for oleogels include proteins (e.g., β-lactoglobulin)
[14][15][14,15], some polymers
[16] and polysaccharides (e.g., ethylcellulose, hydroxypropyl methylcellulose, alginates)
[17][18][17,18].
Polymers that are most commonly used to form oleogels and are also the most promising group are polysaccharides. These complex sugars widely found in various living organisms perform a wide range of functional properties, including energy storage, support cell and tissue function, and are responsible for cell signaling. The structure of the polymer chain is based on monosaccharide units such as fructose and glucose, which come together via a glycosidic bond during the dehydration reaction
[19].
The functionality of polysaccharides is a direct result of their molecular structure, which, depending on the type of monosaccharide, the position, and type of glycosidic bonding, can take both linear and branched forms. Hydroxyl groups (-OH) and other side groups of polymer chain structure influence the spatial configuration of the molecule, which directly affects its ability to form intermolecular hydrogen bonds and intramolecular interactions between chains and other elements. In turn, these features lead to higher-order spatial structures, such as the helix
[20].
An example of a polysaccharide composed of two types of monomers (D-mannuronic acid and L-guluronic acid linked by a β-1,4-glycosidic bond) is alginate originating from seaweed. The structure of alginate results from the arrangement of the individual monomer units and the length of the blocks, which in turn depend on the type of tissue, species, and place of origin of the algae and significantly determine its characteristics as a gelator. The main advantage of using this polysaccharide in oleogelation is the simplicity and low cost of the process and the high mechanical stability and non-toxicity of the resulting gels
[21][22][21,22].
The approach for using the minimally processed natural gum as oleogelators is worth exploring while research reports considering their specific usage are still scarce despite a variety of existing natural gums. The main explored polysaccharides are still cellulose derivatives. However, they are usually modified physically (crystalline or micro cellulose) or chemically (ethylcellulose).
2. Oleogelation with Natural Gums
Gelators (biopolymers), e.g., proteins and polysaccharides used to structure oils, are mostly commercially available, relatively inexpensive, and mostly permitted for use in the food industry
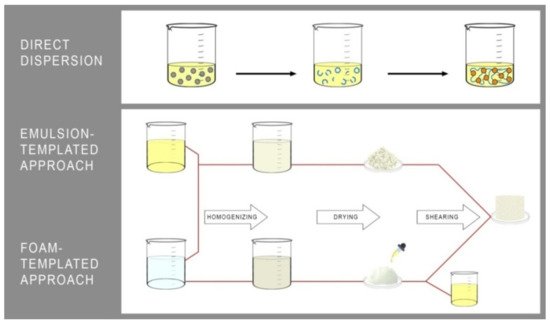
[23][24][25][23,24,25]. Among the methods leading to the oleogels formation, three leading strategies stand out, which are: (1) a direct dispersion of structuring agents
[26] and the indirect ones based on the formation of (2) structural emulsions
[23] or (3) porous foams filled by the liquid oil
[27][28][27,28] (
Figure 2).
Figure 2. The leading strategies used for oleogels manufacturing.
Recently, the increasing interest in using proteins and stabilizing them with polysaccharides as components to form oleogels appeared
[29]. Various polysaccharides are used to stabilize such lipid structures like pectin, sodium alginate, xanthan gum, tara gum, gellan gum, acacia gum, and carob gum. These polysaccharides originate from a variety of sources, i.e., carrageenan, alginate derive from algae, while gellan and xanthan gum are of bacterial origin. Biopolymers such as pectin, tara, carob, and acacia gums are of plant derivatives, while chitin comes usually from crustaceans or fungi
[30].
The molecular structure of individual polysaccharides differs a lot, e.g., alginate, gellan gum, and carrageenan have a linear structure, xanthan, acacia gum, and pectins are hetero-polysaccharides and thus composed of different monomers, while tara and carob gum are galactomannans
[31][32][31,32].
The molecular structure of gelators significantly affects the physical, chemical, and rheological properties of the resulting oleogel, which is related to the accessibility of the molecule fragments that form the contact sites. Wijaya et al. (2019, 2017)
[17][29][17,29] proposed an emulsion method for oil structuring using proteins and polysaccharides while also investigating the effect of pH on the properties and stability of oleogels.
During the lipid structuring, in a first step, water–oil emulsions (from 60% to 80% oil by volume in the emulsion) were prepared stabilized by a matrix consisting of a combination of suitable gelators, in which water was then disposed of during heat treatment to obtain a solid oleogel texture with an increased oil content (above 90%). Studies have shown that using proteins and polysaccharides as gelators results in higher stability of oleogels and slows down the oil release due to their emulsifying and stabilizing properties. However, this depends mainly on the chemical and structural features of the used gelator
[16][17][33][16,17,33].
Vélez-Erazo et al. (2020)
[30] demonstrated that the most stable oleogels after four weeks of storage at 5 °C were structures that based on xanthan gum and tara gum. These formulations had a uniform and creamy appearance throughout the storage period and showed minimal oil loss. Such behavior was due to the properties of these gelators, which have a high viscosity despite having weaker gelling properties than the other polysaccharides used in the experiment. Water–oil emulsions undergo dehydration during mild drying. The resulting polymer network serves as a building block for the dry product, which, through shearing, gives the final oleogel with the desired texture and functional properties
[30].
Some studies show that the ideal way to improve the stability of emulsions is to force the polymers to form colloid complexes. Interactions occurring in such an emulsion between, e.g., polysaccharides and proteins and at the water–oil interface result in better hydration, surface properties (i.e., adsorption, charge, surface tension and film thickness), and a more stable structure. By creating nanocomplexes of gelatin, tannic acid, and linseed gum, Qiu et al. (2018) obtained an oleogel made of the three components using an emulsion method
[34].
The applied tannic acid, which is a derivative of polyphenols, as a natural antioxidant having a large number of hydroxyl groups, interacts with carbonyl groups of polysaccharides and proteins, creating strong covalent and non-covalent interactions. Due to its ability to scavenge, free radicals perform antioxidant functions, thus preventing the oxidation of unsaturated fatty acids in the oleogel. Complexation with tannic acid allows it to accumulate on the surface of the oil droplet and act as a natural crosslinking agent that can manipulate the architecture and improve the antioxidant activity of the emulsion. In addition, the use of linseed gum (an anionic polysaccharide extracted from linseed), characterized by its excellent thickening and emulsifying capacity with good gelling properties, allowed for a more stable oleogel structure (without significant oil escape) with important health benefits
[34].
Furthermore, oleogels based on these complexes were characterized by high thixotropy and hydration. The compact structure of the oleogel was made possible by interfacial adsorbed particles on the inner surface of the oil–water phases (this mechanism resembles the stabilization of Pickering emulsion particles in oil–water systems), which formed an interfacial “coating” and also polymer networks. In the future, oleogels based on these gelators may have applications for the production of foods with controlled rheological and textural properties
[34].
Another method, used as frequently as the emulsion-based one, is based on first producing a porous foam of biopolymers and then saturating the foam with oil and shearing the resulting product with high-energy homogenisation.
The method proposed by Abdollahi et al. (2020)
[28] showed that the addition of xanthan gum increased the viscosity and stability of the foam. Increasing the concentration of xanthan gum multiplied the network density and the hardness, but did not affect the moisture sorption. The oil binding capacity of such oleogels was >92%. The oleogel network produced by the “foam” method can protect edible oil from oxidation during two-month storage. The formation procedure, which makes foam-type oleogels that maintain oil even at high temperatures, may be of interest to researchers looking for solid fat substitutes in food, e.g., in pastry products
[28].
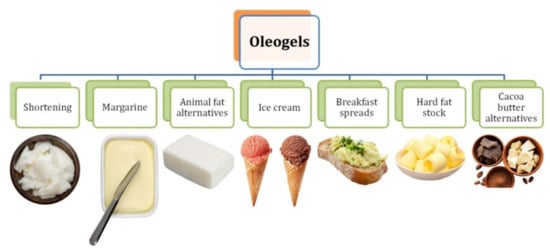
3. Food Applications of Oleogels
The growing interest in oleogelation is constantly observed
[10]. Oleogels may in the future be used in the food, pharmaceutical, cosmetic, and petrochemical industries
[35][93]. One of the possible applications in the food industry that has been previously mentioned is undoubtedly to reduce the use of solid fats rich in saturated fatty acids and trans isomers and also to minimise the migration of edible oils in multi-ingredient food products
[36][94] (
Figure 3).
Figure 3. Products dedicated for possible oleogel incorporation.
The oleogels role in meat products would be to partially replace beef fat, with which they share a similarity in fat particle size
[3]. Depending on the biopolymer used, which determines the following properties of the oleogel obtained. They can be used as fatty ingredients in various food products. In all likelihood, structured edible oils will find a variety of industrial applications in the future and will thus draw even more interest from the scientific community in this topic.
Oil emulsions could become an alternative solution in pastry or confectionery products, and the health benefits of avoiding the harmful trans fats will increase consumer interest. One recent study shows that using oleogels based on natural biopolymers such as pectin has a positive effect on confectionery and proved that adding 50% (
v/
v) of the dispersed hydrogel to the other chocolate ingredients produced a low-fat chocolate product resistant to melting at 80 °C
[37][95]. Previous studies on applications and potential uses in food products presented results in which oleogels based primarily on structures using oleogelators such as ethylcellulose were used
[38][39][96,97].
In the pastry industry, oleogels could substitute so-called shortening agents, which contain animal fats in their composition. Luo et al. (2019) demonstrated that adding oleogels consisting of lemon pectin and oil to dough resulted in fewer bubbles during baking than those where traditional butter was used, most likely due to the absence of fat crystals and emulsifiers in the oleogels.
Additionally, it was found that as the pectin concentration in the oleogel matrix increased, the number of air bubbles in the dough decreased. This is a consequence of the dough’s mechanical strength, which prevents the introduction of air during the mixing process. The internal structure became firmer and more compact when the pectin concentration in the oleogels was increased to 4.5% (
m/
v). The samples for which the pectin concentration in oleogels was 1.5% and 2.5% (
m/
v) showed good dough quality
[40][52].
Texture analysis and sensory evaluation of the cakes showed that the hardness of the baked goods where oleogels were used is higher than in those where butter was used, without a significant difference in elasticity in all samples and is similar to the results obtained by other researchers
[41][42][88,98]. This was due to the dough structure produced in which pectin formed a network, further increasing the hardness of the baked goods
[40][52].
Another interesting finding was that all baked goods samples, both where butter and oleogels were used, did not differ in aroma. Replacing solid fats with oleogels in baked goods seems on the surface to be a simple undertaking that would reduce saturated fatty acid content. However, formulating food products without the addition of solid fats is quite a difficult task. These are primarily responsible for the required structure, texture and, above all, taste sensation. Therefore, partial replacement of butter with oleogels is a promising method to reduce saturated fatty acids and trans fatty acids while maintaining the desired physical properties of baked goods
[40][52].
The above-presented research using more natural polysaccharides, such as, e.g., alginates and pectins, is a novel endeavor and opens new insights into applications using such oleogels in low-fat foods
[40][37][52,95]. Additionally, in the future, these oleogels may find broader application in food and respond to increasingly demanding consumers and food manufacturers promoting healthy and organic nutrition.