A bacterium becomes resistant due to the transfer of genes encoding antibiotic resistance. Bacteria constantly mutate; therefore, their defense mechanisms change constantly. Nanotechnology plays a key role in antimicrobial resistance due to materials modified at the nanometer scale, allowing large numbers of molecules to assemble to have a dynamic interface. These nanomaterials act as carriers, and their design is mainly focused on introducing the temporal and spatial release of the payload of antibiotics. In addition, they generate new antimicrobial modalities for the bacteria, which are not capable of protecting themselves.
1. Introduction
The application of scientific knowledge to manipulate and control matter predominantly in the nanoscale to make use of size and structure dependent properties, and phenomena distinct from those associated with individual atoms or molecules, or extrapolation from larger sizes of the same material
[1], which consist of the ability to synthesize, manipulate, and modify materials below 100 nanometers
[2][3][4][5][2,3,4,5], has postulated as nanotechnology a fundamental discipline in scientific and technological advances in different areas, such as medicine and the pharmaceutical industry, to provide solutions for various existing problems in these areas.
It is considered that a nanostructured material must have dimensions within 1 to 100 nm
[5][6][5,6]. However, in medicine, these values can range up to 200 nm in diameter
[7]. Among these materials, the use of nanoparticles (NPs) stands out metallic, bimetallic, metal oxide, and magnetic
[8][9][10][8,9,10].
The use of metallic and metal oxide NPs has been increasing due to the chemical and physical intrinsic properties acquired by NPs synthesized
[11]. Depending on their application, the optical, catalytic, and electrical behavior, mechanical and chemical stability
[12][13][12,13], as well as morphology and particle size, can be controlled
[14], which makes them suitable for the pharmaceutical industry.
Metallic NPs allow the possibility of interacting at biomolecular levels
[15]. This improves detection, treatment, and monitoring of pathologies, through the specific targeting of cells and tissues. In addition, it helps with the administration of drugs, evaluation of diseases, and treatment of degenerative conditions
[16], which makes them promising materials for directing chemotherapeutic drugs.
The pharmaceutical and medical industry was presented with one of the biggest problems worldwide since 2015, when the World Health Organization (WHO) declared the increase of antimicrobial resistance by pathogenic bacteria as a priority to study. The WHO published in 2017 a list of pathogens with the highest risk worldwide (
Table 1)
[17]. These bacteria are resistant to antibiotics and have been classified based on various criteria, such as mortality and resistance prevalence, among others, classifying them as critical, high, and medium priority
[18].
Table 1. The official list of pathogen bacteria with declared priority by the WHO. Adapted with permission from WHO (permission 387722)
[17].
|
| Priority |
|
| Pathogenic Bacteria |
|
| Antibiotics for Which There is Resistance |
|
|
| Critical |
|
| Acinetobacter baumannii |
|
| Carbapenem |
|
|
| Pseudomonas aeruginosa |
|
|
| Enterobacteriaceae |
|
|
| Mycobacteria |
|
| Carbapenem and 3rd generation cephalosporins |
|
|
| Mycobacterium tuberculosis |
|
| 3rd generation cephalosporins |
|
|
| High |
|
| Enterococcus faecium |
|
| Vancomycin and methicillin |
|
|
| Staphylococcus aureus |
|
|
| Helicobacter pylori |
|
| Vancomycin |
|
|
| Campylobacter |
|
| Clarithromycin |
|
|
| Salmonella spp. |
|
| Fluoroquinolones |
|
|
| Neisseria gonorrhoeae |
|
| 3rd generation fluoroquinolone |
|
|
| Medium |
|
| Streptococcus pneumoniae |
|
|
|
| Haemophilus influenza |
|
| Non-sensible to penicillin |
|
|
| Shigella spp. |
|
| Ampicillin and fluroquinolones |
|
Antimicrobials are organic small molecules (they vary in size at angstrom level) that prevent the development of pathogenic microorganisms, which are generally used in bacteria. Antimicrobial agents can be divided into three groups according to their characteristics: disinfectants, antiseptics, and those for clinical-therapeutic use
[19]; the latter are known as antibiotics capable of reducing and controlling the presence of bacteria that have invaded the patient’s body.
Before the use of antibiotics, the mortality rate caused by pathogenic bacteria was high
[18]. However, at the end of the 19th century and the beginning of the 20th century, antibiotics began to be studied. This led to the discovery of penicillin, using it clinically in 1930, together with sulfamide. These antibiotics were effective against Gram-positive and Gram-negative bacteria
[20]. Unfortunately, the capacity of these antibiotics to treat infectious diseases caused by bacteria has not been enough, and this represents a danger for the population
[21].
Excessive and uncontrolled use of antibiotics have generated resistance to antimicrobials by bacteria, as well as the spread of resistant bacteria in hospitals, and have become some of the most important problems in recent years
[22].
In the United States alone, according to the Centers for Disease Control and Prevention (CDC), the first report on threats by antimicrobial resistance was published in 2013. This report mentions that, in the U.S., at least 2 million people contract an infection by bacteria resistant to antibiotics, and at least 23,000 people died because of this
[23]. However, in 2019, an increase to 2.8 million infected patients by resistant bacteria was reported, of which more than 35,000 people died every year. Thus, producing an economic impact of more than 4.6 billion dollars annually in the United States alone
[24].
2. Antibiotics
Antibiotics are antimicrobial drugs capable of reducing and controlling the presence of bacteria that have invaded the tissues of a subject. Antibiotics are grouped into classes according to their chemical structure, effect, spectrum, and action mechanism
[25][26][27][28][26,27,28,29].
2.1. By Chemical Structure
According to their chemical structure, the antibiotics can be grouped as β-lactam antibiotics, macrolides, aminoglycosides, and tetracycline antibiotics
[29][30].
2.2. By Effect
This group corresponds to those that caused the death of the most sensitive microorganism, in the bacteria growth phase (bactericidal) or those that inhibit bacterial growth (bacteriostatic)
[30][31].
2.3. By Spectrum
This classification is divided into three branches: the broad spectrum, the limited spectrum, and the narrow spectrum. When talking about broad spectrum antibiotics, it emphasizes that the drug acts on a wide range of bacteria which can be Gram-positive and Gram-negative. Limited spectrum antibiotics are those acting only against Gram-positive or Gram-negative cocci, as well as Gram-positive bacilli and spirochetes, as is the case with penicillin. Lastly, narrow spectrum antibiotics, which attack only a very small sector of bacteria
[31][32].
2.4. By the Mechanism of Action
The classification of antibiotics according to their mechanism is divided into four main ones which consist of inhibiting cell wall synthesis, protein synthesis, nucleic acid synthesis, and antimetabolites.
According to Murray et al.
[31][32], in Medical Microbiology, antibiotics for inhibiting cell wall synthesis are the penicillins, cephalosporins, carbapenems, and cephamycins, since they bind to penicillin-binding proteins (PBP) and enzymes responsible for peptidoglycan synthesis. On the other hand, vancomycin, such as the other antibiotics, usually damages the cell wall; however, the mechanism of vancomycin is to inhibit the cross-linking of the peptidoglycan layers, such as cycloserine, thus causing cell death. Bacitracin is responsible for inhibiting the cytoplasmic membrane of the bacterium, as well as the movement of peptidoglycan precursors. Antibiotics of the polymyxin family often damage the bacterial membrane
[26][27].
In the case of inhibiting protein synthesis, in Murray et al.
[31][32], drugs, such as aminoglycosides, are used because they are responsible for the premature release of peptide chains from the 30S ribosome. Likewise, tetracyclines damage proteins by preventing polypeptide elongation in the 30S ribosome. Antibiotics of the macrolide, ketolide, clindamycin, oxazolidinone, and streptogramins groups are responsible for preventing protein synthesis and polypeptide elongation on the 50S ribosome.
The groups of quinoline, rifampicin, rifabutin, and metronidazole are antibiotics that usually impair nucleic acid synthesis, i.e., their mechanism of action is given by binding the DNA gyrase subunit, preventing transcription by binding DNA dependent RNA polymerase
[31][32].
Finally, according to Murray et al.
[31][32], antibiotics of the sulfonamide, dapsone, and trimethoprim families are responsible for damaging the metabolic pathways of bacteria, as they tend to inhibit dihydropteroate synthase and dihydrofolate reductase, which triggers the folic acid synthesis disruptions.
3. Antimicrobial Resistance
Antimicrobial resistance (AMR) is a natural phenomenon of bacteria
[32][33] that develops thanks to its intrinsic evolutionary nature, as well as its easy and rapid adaptability to various environments
[33][34]. However, the abuse and excessive use of antibiotics has given bacteria the ability to create greater resistance to antimicrobials
[34][35][36][37][38][39][40][41][35,36,37,38,39,40,41,42], which translates into the lack of ability of antibiotics to inhibit the growth of pathogens
[42][43]. This has alerted public health organizations worldwide and has led to major regulated and controlled antibiotic administration measures, to improve treatments in patients
[43][44][44,45].
Resistance levels can vary greatly according to the groups of bacteria. Susceptibility and resistance are generally measured as a function of the minimum inhibitory concentration (MIC), which is the minimum concentration of the drug that will inhibit bacteria growth
[45][46]. Susceptibility is a range of the average MICs for any given drug in the same bacterial species. If the average MIC for a species is in the resistant part of the range, such species are considered to have intrinsic resistance to that drug. Bacteria can also acquire resistance genes from other related organisms, and the level of resistance will vary according to the species and the genes acquired
[46][47][47,48].
When referring to intrinsic resistance, it means that bacteria can be naturally resistant to some antibiotics
[48][49], and this is due to the particular characteristics of each bacterium, which depend on its structure and function
[49][50]. That is when the composition and chemical structure of the antibiotic is unable to penetrate or react with the structure of the bacterial membrane. An example of this type of resistance is
Pseudomonas aeruginosa because it has a membrane with low permeability, and this makes it naturally resistant to most antimicrobials
[50][51][52][53][51,52,53,54].
On the other hand, bacteria can also acquire various AMR mechanisms either by gene transfer mechanisms or by biochemical mechanisms
[54][55]. Among the genetic mechanisms are the chromosomal and extra chromosomal mutation
[55][56], which is called acquired resistance
[56][57]. This type of AMR is due to the evolutionary pressure that bacteria develop against the attack of antibiotics, changing their genome through genomic mutation or by cellular selection. This exchange of genes is carried out through transformation, transduction, or conjugation
[31][32].
Mutations develop after excessive exposure to antibiotics, which provides pathogens with strong resistance mechanisms and, therefore, greater virulence, which complicates drug treatment against bacterial infections and can result in a greater complication
[47][48].
The biochemical mechanisms of AMR can occur due to the modification of the antibiotic bacterial target, but the enzymes that modify antibiotics are only capable of affecting certain antimicrobials
[54][57][55,58]. The enzymatic inactivation of antimicrobial drugs is when there are mutations in genes that can encode porin proteins around the bacterial membrane to slow down the action of antibiotics. Another biochemical mechanism is the flow pump system that can expel antimicrobial drugs without being damaged and the reduction of intracellular concentrations because of the decrease in permeability and flow
[58][59][59,60].
AMR can be caused not only by chromosomal or extrachromosomal mutations but also by cross-transfer. This means that a bacterium resistant to one antibiotic or a family of antibiotics, in particular, when encountering another antibiotic or another group of antibiotics with a similar chemical structure, will likely recognize such structure and create this immunity to this new family of antimicrobials
[60][61][62][61,62,63].
4. Nanotechnology Applied to Antimicrobial Resistance
Nanotechnology currently plays a key role in scientific and technological advances in medicine and the pharmaceutical industry, this concerns the use of materials controlling their size and shape
[2]. In these senses, the nanoparticles (NPs) are particulate materials on a nanometric scale that allow modifying both the physical and chemical properties of materials, as well as their morphology and size, which ranges from 1 to 100 nm
[63][64][65][113,114,115]. The smaller and more spherical the NPs are, the greater the surface-volume ratio is achieved, which helps to enhance the chemical and biological activities of the NPs
[66][67][116,117].
The NPs which have been used for different applications
[68][69][118,119], such as drug administration, photo ablation therapy, biological imaging, applications in biosensors, and even as an alternative to reduce antimicrobial resistance, have stood out with great relevance
[70][71][72][120,121,122].
These applications include the use of NPs as antimicrobial components on advanced materials for medical devices as catheters walls, valves, stents, and a surface that could be found inside or outside the body or could be designed for in vivo therapies. The development of advanced materials includes the use of Fe
3O
4 functionalized with chitosan and lysozyme to produce a coating for producing biofilm-resistance surfaces
[73][123]. The NPs designed for in vitro applications include their use as a drug administration; in these applications, the NPs can load with different molecules as an essential oil, such as the ZnONPs have been loaded with
Citronella essential oil
[74][124] or Oxide-Silica Core-Shell with essential oil
[75][125], both with antimicrobial activity. In addition, Fe
2O
3NPs have been used as carrier paclitaxel and β-cyclodextrin
[76][126] or PdNPs capping with polyvinylpyrrolidone load with quercetin
[64][114], and Silica Core-Shell Au
[77][127] for Cancer Therapy.
NPs can be classified as metallic, metal oxide, bimetallic, and magnetic
[8][78][79][8,128,129]. It has been demonstrated that this type of particle can obtain antimicrobial properties so that, when increasing the surface area of the particles, a greater contact area with microorganisms is generated
[15][80][81][82][15,130,131,132], thus enhancing its antimicrobial activity.
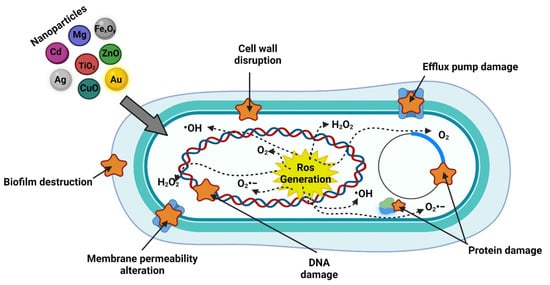
As well as acting as antibacterial agents that can cause alterations in the bacterial membrane, metallic, bimetallic, and metal oxide NPs usually produce reactive oxygen species (ROS) by releasing metallic ions that alter the cellular components of bacteria
[83][84][133,134], and the smaller nanoparticles are the damage created by them will be greater because they tend to be better absorbed on the bacterial surface. This is because, in some cases, the NPs have a positive surface charge that facilitates the union with the negative charge on the surface of bacteria
[79][85][86][129,135,136]. Likewise, the photodynamic and photothermic effects of NPs generate a greater impact as antimicrobial agents (
Figure 1), which is directly related to the release of metallic ions and ROS
[6].
Figure 1.
Action mechanism of inorganic nanoparticles. Created with
(accessed on 17 November 2021).
NPs sizes are largest than antibiotics which allow the use of these as carriers of antibiotics or other small molecules as antibodies or chemotherapeutic agents
[76][126].