Mycosporine-like amino acids (MAAs), are secondary metabolites, first reported in 1960 and found to be associated with the light-stimulated sporulation in terrestrial fungi. MAAs are nitrogenous, low molecular weight, water soluble compounds, which are highly stable with cyclohexenone or cycloheximine rings to store the free radicals. Microalgae are considered as a good source of different kinds of MAAs, which in turn, has its own applications in various industries due to its UV absorbing, anti-oxidant and therapeutic properties. Microalgae can be easily cultivated and requires a short generation time, which makes them environment friendly source of biomolecules such as mycosporine-like amino acids. Modifying the cultural conditions along with manipulation of genes associated with mycosporine-like amino acids biosynthesis can help to enhance MAAs synthesis and, in turn, can make microalgae suitable bio-refinery for large scale MAAs production.
- mycosporine-like amino acids
- microalgae
- Photoprotective compounds
1. Structure of Mycosporine-like Amino Acids (MAAs)
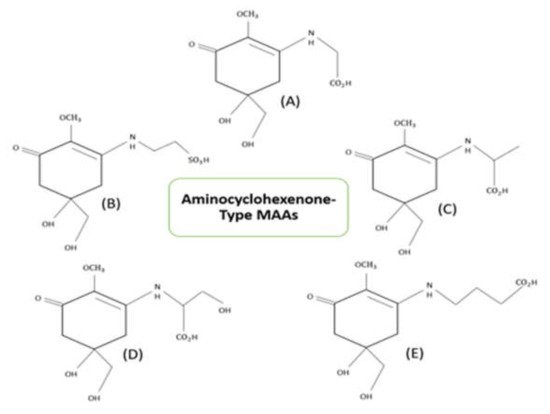
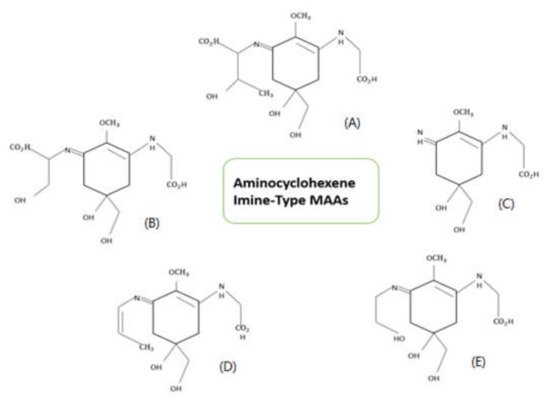
2. Properties of Mycosporine-like Amino Acids (MAAs)
References
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646.
- Gorbushina, A. Microcolonial Fungi: Survival Potential of Terrestrial Vegetative Structures. Astrobiology 2003, 3, 543–554.
- Oren, A. Mycosporine-like amino acids as osmotic solutes in a community of halophilic cyanobacteria. Geomicrobiol. J. 1997, 14, 231–240.
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 105–114.
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502.
- Rosic, N.N. Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar. Drugs 2019, 17, 638.
- Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-Like Amino Acids as Multifunctional Secondary Metabolites in Cyanobacteria: From Biochemical to Application Aspects. Bioact. Nat. Prod. Part K 2018, 59, 153–194.
- Gacesa, R.; Lawrence, K.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.; Wells, G.; Young, A.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44.
- Dunlap, W.C.; Shick, J.M. Review-ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: A biochemical and environmental perspective. J. Phycol. 1998, 34, 418–430.
- Shick, J.M.; Dunlap, W.C. Mycosporine-Like Amino Acids and Related Gadusols: Biosynthesis, Accumulation, and UV-Protective Functions in Aquatic Organisms. Annu. Rev. Physiol. 2002, 64, 223–262.
- Rozema, J.; Björn, L.; Bornman, J.; Gaberščik, A.; Häder, D.-P.; Trošt, T.; Germ, M.; Klisch, M.; Gröniger, A.; Sinha, R.; et al. The role of UV-B radiation in aquatic and terrestrial ecosystems—an experimental and functional analysis of the evolution of UV-absorbing compounds. J. Photochem. Photobiol. B Biol. 2002, 66, 2–12.
- Sinha, R.; Klisch, M.; Gröniger, A.; Häder, D.-P. Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J. Photochem. Photobiol. B Biol. 1998, 47, 83–94.
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–172.
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10.
- Böhm, G.A.; Pfleiderer, W.; Böger, P.; Scherer, S. Structure of a Novel Oligosaccharide-Mycosporine-Amino Acid Ultraviolet A/B Sunscreen Pigment from the Terrestrial Cyanobacterium Nostoc commune. J. Biol. Chem. 1995, 270, 8536–8539.
- Sinha, R.P.; Häder, D.-P. UV-protectants in cyanobacteria. Plant Sci. 2008, 174, 278–289.
- De La Coba, F.; Aguilera, J.; Korbee, N.; De Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55.
- Furusaki, A.; Matsumoto, T.; Tsujino, I.; Sekikawa, I. The Crystal and Molecular Structure of Palythine Trihydrate. Bull. Chem. Soc. Jpn. 1980, 53, 319–323.
- Ferroni, L.; Klisch, M.; Pancaldi, S.; Häder, D.-P. Complementary UV-Absorption of Mycosporine-like Amino Acids and Scytonemin is Responsible for the UV-Insensitivity of Photosynthesis in Nostoc flagelliforme. Mar. Drugs 2010, 8, 106–121.
- Zhang, Z.; Tashiro, Y.; Matsukawa, S.; Ogawa, H. Influence of pH and temperature on the ultraviolet-absorbing properties of porphyra-334. Fish. Sci. 2005, 71, 1382–1384.
- Conde, F.; Churio, M.; Previtali, C. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000, 56, 139–144.
- Arbeloa, E.M.; Uez, M.J.; Bertolotti, S.G.; Churio, M.S. Antioxidant activity of gadusol and occurrence in fish roes from Argentine Sea. Food Chem. 2010, 119, 586–591.
- Hirata, Y.; Uemura, D.; Ueda, K.; Takano, S. Several compounds from Palyfhoa tuberculosa (Coelenterata). Pure Appl. Chem. 1979, 51, 1875–1883.
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.-P. Mycosporine-like amino acids in the marine red alga Gracilaria cornea—effects of UV and heat. Environ. Exp. Bot. 2000, 43, 33–43.
