Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by ANDREA MONTAGNANI and Version 2 by Camila Xu.
RAASi therapy (ACEi, ARB, MRA, and ARNi therapy) is the cornerstone therapy for patients with heart failure with reduced ejection fraction (HFrEF).
- hyperkalemia
- Patiromer
- sodium zirconium cyclosilicate
1. Description of the Condition
RAASi therapy (ACEi, ARB, MRA, and ARNi therapy) is the cornerstone therapy for patients with heart failure with reduced ejection fraction (HFrEF). International guidelines recommend RAAS inhibitors because of strong evidence [1][2][1,2]. Even so, there are some barriers to the implementation of this therapy, including hypotension, kidney dysfunction, and hyperkalemia (HK) [3]. HK is defined as high serum potassium (K+) levels. There is no universal cutoff for HK, but a level of 5.0 mEq/L is common [4]. HK is a life-threatening condition that leads to significant morbidity and mortality. A recent evaluation of medical records demonstrated an increase in all-cause mortality both with K+ values below the normal range and values higher than 5.5–6.0 mEq/L, with a clear effect of comorbidities, such as diabetes, heart failure, and CKD, considered individually or together [5]. In three pivotal clinical studies examining the efficacy of RAAS inhibitors in heart failure, HK occurred in these patients at a non-negligible frequency, although patients with a high risk of HK (i.e., with impaired renal function) were excluded from the studies [6][7][8][6,7,8].
The latest ESC guidelines recommend that RAAS inhibitors should be administered at the maximum recommended dose in order to achieve a positive effect on mortality in patients with HFrEF [1]. The protective effect of RAAS inhibitors on mortality in patients with heart failure is maximized when used in combination, but at the same time, a higher risk of HK, and therefore a worse outcome, is caused by using a combination with a sub-optimal dose of RAASi. In addition, patients who appear to benefit from the administration of RAAS inhibitors also have a higher risk of HK [9]. Recently, Rossignol et al. published data from 9222 patients with chronic heart failure in the ESC-HFA-EORP Heart Failure Long-Term Registry, confirming the association between HK and mortality. However, when the same data were adjusted for RAAS inhibitor discontinuation, HK was no longer associated with mortality, suggesting that HK may be a risk marker for RAAS inhibitor discontinuation rather than a risk factor for poorer outcomes [10]. In an unpublished survey that the Federation of Hospital Internists (FADOI) carried out among approximately 300 Italian internists in 2020, RAAS inhibitors were discontinued by 80% of physicians when potassium was more than 5.5 mEq/L or down-titrated when potassium serum values were higher than 5.0 mEq/L. This conservative and cautious approach seems to be common since HK is correlated with higher mortality; however, it induces a lack of optimization of therapy in patients with heart failure and/or CKD who are treated with RAAS inhibitors, with a consequent increase in mortality [11].
2. Description of the Intervention
Two new drugs have been developed to treat HK: Patiromer and sodium zirconium cyclosilicate (SZC). Patiromer is a spherical, non-resorbed, metal-free, cross-linked fluoroacrylate polymer [12]. Sodium zirconium cyclosilicate is a non-absorbed, non-polymeric inorganic powder with a uniform microporous structure [13].
3. How the Intervention Might Work
Most potassium is eliminated through the kidneys, but 5–10% is eliminated in the colon. Both Patiromer and SZC remove potassium by exchanging cations (calcium and sodium for Patiromer and SZC, respectively) for potassium in the gastrointestinal tract, binding potassium and increasing its fecal excretion. Patiromer was developed to provide a higher potassium binding capacity compared with the polystyrene sulfonate polymer. It has improved physical properties, such as a low swell ratio that allows for minimal water uptake, and, importantly, Patiromer uses calcium rather than sodium as the exchange cation. Patiromer was completely ionized for optimal ion exchange at the physiological pH of the large intestine, where the potassium concentration in the gastrointestinal tract was the highest [12]. Sodium zirconium cyclosilicate is highly selective for potassium ions in vitro, even in the presence of other cations, such as calcium and magnesium. Sodium zirconium cyclosilicate traps potassium throughout the gastrointestinal tract (GI) and reduces the concentration of free potassium in the GI lumen, thereby lowering serum potassium and increasing fecal potassium excretion to resolve HK [13].
4. Effects of Interventions
NPBs seemed to have an effect on the optimization of MRA therapy, with a risk ratio (95% CI) of 1.24 (1.09, 1.42); there was no heterogeneity (I2 = 0%, moderate certainty evidence). Patiromer seemed to have an effect on MRA optimization; in two studies with 232 patients, the risk ratio (95% CI) was 1.25 (1.08, 1.45) with I2 = 0 (high certainty evidence). ZSC seemed to have no effect on MRA optimization, as shown by one study with 176 patients (risk ratio (95% CI)), 1.19 (0.89, 1.59) (low certainty evidence)) (Figure 13). The subgroup analysis did not show heterogeneity between the subgroups (I2 = 0%).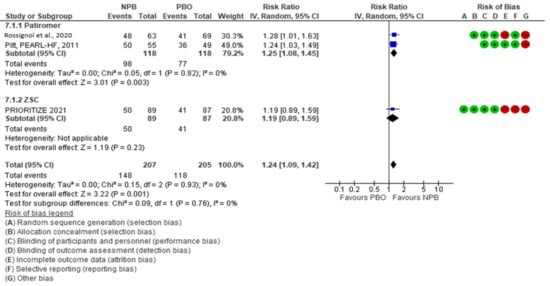
Figure 13. MRA optimization.
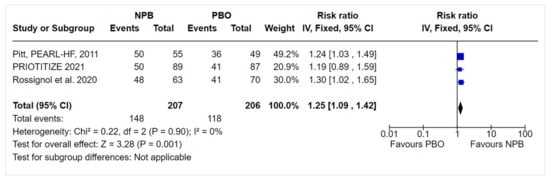
Figure 24. Sensitivity analysis.
