Fungal phytopathogens are a growing problem all over the world; their propagation causes significant crop losses, affecting the quality of fruits and vegetables, diminishing the availability of food, leading to the loss of billions of euros every year. To control fungal diseases, the use of synthetic chemical fungicides is widely applied; these substances are, however, environmentally damaging. Marine algae, one of the richest marine sources of compounds possessing a wide range of bioactivities, present an eco-friendly alternative in the search for diverse compounds with industrial applications.
- algae phenols
- antifungal activity
- bioactive compounds
- brown algae
- crop losses
- fungal membrane disruption
- fungal resistance
- lipophilic compounds
- macroalgae metabolites
- plant pathogens
1. Introduction
Plant pests pose a paramount problem that has been increasing in recent years. The exact production losses due to these phytopathogens are hard to quantify but it is estimated that plant pests account for 20–40% of annual crop production losses [1][2][1,2], at a cost of more than 185 billion euros [3]. Included among these pests, fungal pathogens are one of the most damaging agents in plants, accounting for the devastation of myriad fruits and crops, which results in vast economic losses [4], and ultimately reduces food availability for a continuously increasing world population [5][6][5,6]. In fact, diseases provoked by fungi or related microorganisms have already caused starvation scenarios, such as the Irish Potato Famine in the 19th century, caused by a fungal-like oomycete, which led to a million of deaths, mass emigration, and economical and political crisis in Ireland [7][8][7,8]. Phytopathogenic fungi were also responsible for the baring of landscapes caused by Dutch elm blight and chestnut blight [8] and the complete ruin of 30% of world food crops in 2012 [3]. Currently, it is predicted that phytopathogenic fungi are responsible for about 80% of plant diseases [9][10][11][9,10,11], for which the absence of control can lead to disastrous global crop losses [6][12][6,12]. Even the remaining crops, potentially infected but without symptoms, can raise concerns about consumption safety [13]. Moreover, current and forecasted climatic change scenarios, leading to the increase of temperature and humidity, are crucial conditions promoting the dispersion and development of phytopathogenic fungi, giving cause for extra concerns [12][14][12,14].
The regular application of agrochemicals with antimicrobial properties is the most effective method against these microbial phytopathogens, but it is expensive and environmentally harmful, prevailing in the ecosystem and damaging it [15][16][15,16]. Every year, farmers spend more than 6 billion euros on such products to control the microbial infections, which represents a quarter of the costs for agricultural purposes [17]. For sustainability reasons, novel alternative methods have been sought that will have the same effectiveness, improve agricultural techniques, and enhance food production, ensuring the quality and security of food [18]. Several techniques and methodologies have been tested to minimize plant and financial losses either by directly targeting the microbial phytopathogens or by preventive measures, conferring resistance to the plant hosts. The laboratory manipulation of synthetic compounds to increase the effectiveness of products [19] or the introduction of “site-specific fungicides” [20] to control the most problematic and common microbial pathogens, have been suggested. Nevertheless, these products remain inefficient due to the great genetic resources and adaptative abilities of phytopathogens, which allow them to acquire resistance and overcome the efficiency of these types of products [20][21][20,21]. The biocontrol technique, characterized by the introduction of an antagonist microbial organism, harmless to the host but damaging for the phytopathogen [14], has been tested in vitro [2][22][23][24][25][26][2,22,23,24,25,26] and shown a great potential in field applications [2]. This methodology is characterized by the absence of chemicals, providing a viable and sustainable agriculture [27]. Although some limitations associated with the establishment and maintenance of biocontrol agents have been identified [2], including their interaction with the plant microbial community [28], the continuous stress conditions provoked in the host plant, the inconsistent results among tests [14][29][30][14,29,30], and the poor effectiveness compared to chemical fungicides, are factors which could and should be improved [29][30][31][29,30,31]. Though their potential can be enhanced through their combination with chemical interventions [28][32][28,32], this fails to solve the harm these compounds pose to the environment. The exploitation of genetic manipulation to alter the plant host genome with the insertion of resistance genes [33] was quickly shown to be ineffective against non-target phytopathogenic microorganisms and/or the emergence of new microbial races [15]. Therefore, the continuous search for biodegradable natural compounds, eco-friendly and effective against phytopathogenic microorganisms, is paramount [34], promising as it does to enhance food production and ensure the quality and security of agricultural products [18].
Marine habitats have been increasingly investigated due to the potential of bioactive products synthesized by the micro- and macro-organisms inhabiting them [35] being used in medicine and industry [36]. Seaweeds are one of the most attractive sources of bioactive substances due to their unique and diversified production of phenolic compounds, polysaccharides, fatty acids, and pigments. It is known that macroalgal applications have the potential to go beyond the ongoing uses in cosmetics, agricultural fertilizers, and the food industry [37]. Marine algae have revealed interesting compounds with antibiotic activity against pathogenic bacteria and fungi. Polysaccharides, polyphenols, carotenoids, proteins, peptides, sterols, terpenes, and fatty acids, among others, are the main constituents of algae that are associated with the antimicrobial properties of seaweed extracts [38][39][40][38,39,40]. Moreover, some of these algae compounds are capable of stimulating the natural defences of plants and promoting their resistance against microbial attacks, exhibiting a priming potential [39][41][39,41].
Considering the problems referred to above and the constant reduction of the effectiveness of available eco-friendly methodologies, given the promising results of in vitro assays, macroalgae constitute a source of diverse and natural compounds with antimicrobial potential against phytopathogenic fungi. Given this framework, the present review focuses on the potential of macroalgae-derived products, aiming to combine the available information regarding the potential/activity of fungal phytopathogen inhibition, while trying to clarify/link some “compound mode-of-action” and provide help and insights for future research into antimicrobial products derived from seaweeds.
2. Macroalgae Potential in the Eradication of Fungal Infections in Plants
2.1. Phytopathogenic Fungi
| Fungal Genera | Host Plant | References |
|---|---|---|
| Alternaria | Fruit plants, such as tomato (Lycopersicon esculentum) and apple (Malus domestica) | [49][57][58][59] |
| Aspergillus | Seeds, nuts, and fruits of a wide range of plant species | [57][58][60][61][62] |
| Botrytis | Wide range of plant hosts | [57][63][64] |
| Colletotrichum | Mediterranean plants and trees (fruits), tropical species and vegetables | [42][47][65][66][67][68][69] |
| Fusarium | The broad range of hosts include mono- and dicotyledons in greenhouses, cereals crops, and other plant species, such as tomato, upland cotton (Gossypium hirsutum), banana (Musa sp.), and plants belonging to the Brassicaceae family | [42][52][57][63][64][70][71][72][73][74][75] |
| Penicillium | Fruits and vegetables | [57][58][76][77] |
| Puccinia | Wheat crops (Triticum aestivum) | [42][47][64][78] |
| Rhizoctonia | Root pathogen of a wide range of hosts, including tomato, soybean (Glycine max), pepper (Capsicum annuum), eggplant (Solanum melongena), watermelon (Citrullus lanatus), upland cotton, sunflower (Helianthus annuus), rice (Oryza sativa), and potato (Solanum tuberosum) |
[32][57][71][72][73][74][75][79][80] |
| Rhizopus | Brassicaceae plants | [57][70] |
2.2. Macroalgae Potential against Phytopathogenic Fungi
2.2.1. In vitro Antifungal Potential
| Phytopathogenic Fungi | Host Species | Methodology | Reference |
|---|---|---|---|
| Alternaria alternata | Hormophysa cuneiformis | Agar diffusion assay/Broth microdilution assay | [56] |
| Ulva lactuca | Disc diffusion technique | [58] | |
| Aspergillus fumigatus | Anthophycus longifolius | Well diffusion technique | [51] |
| Osmundea pinnatifida | Radial growth inhibition | [117] | |
| Aspergillus niger | Anthophycus longifolius | Well diffusion technique | [51] |
| Ulva lactuca | Disc diffusion technique | [58] | |
| Aspergillus terreus | Anthophycus longifolius | Well diffusion technique | [51] |
| Botrytis cinerea | Dictyopteris polypodioides | Agar diffusion technique | [63] |
| Cladosporium herbarum | Hormophysa cuneiformis | Agar diffusion assay/Broth microdilution assay | [56] |
| Colletotrichum acutatum | Dictyota dichotoma | Disc diffusion technique | [65] |
| Dictyota implexa | Disc diffusion technique | [65] | |
| Dictyota spiralis | Disc diffusion technique | [65] | |
| Colletotrichum falcatum | Caulerpa racemosa | Poisoned food technique | [66] |
| Hydropuntia edulis | Poisoned food technique | [66] | |
| Sargassum myricocystum * | Poisoned food technique | [66] | |
| Colletotrichum gloeosporioides | Hypnea musciformis | Disc diffusion technique | [67][68] |
| Kappaphycus alvarezii | Poisoned food technique | [69] | |
| Laurencia dendroidea | Disc diffusion technique | [67] | |
| Ochtodes secundiramea | Disc diffusion technique | [67][68] | |
| Palisada flagellifera | Disc diffusion technique | [68] | |
| Pterocladiella capillacea | Disc diffusion technique | [67] | |
| Colletotrichum musae | Hypnea musciformis | Poisoned food technique | [67] |
| Laurencia dendroidea | Poisoned food technique | [67] | |
| Ochtodes secundiramea | Poisoned food technique | [67] | |
| Padina gymnospora | Poisoned food technique | [67] | |
| Pterocladiella capillacea | Poisoned food technique | [67] | |
| Fusarium culmorum | Fucus vesiculosus | Inhibition of mycelial growth/Macroconidia germination inhibition | [57] |
| Fusarium graminearum | Dictyopteris polypodioides | Agar diffusion technique | [63] |
| Fusarium moniliforme | Botryocladia leptopoda | Test tube in agar | [96] |
| Dictyota hauckiana | Test tube in agar | [96] | |
| Fusarium oxysporum | Asparagopsis taxiformis | Well diffusion technique | [118] |
| Calliblepharis floresii * | Poisoned food technique | [52] | |
| Caulerpa chemnitzia | Poisoned food technique | [52] | |
| Caulerpa racemosa | Poisoned food technique | [52] | |
| Caulerpa scalpelliformis | Poisoned food technique | [52] | |
| Caulerpa taxifolia | Poisoned food technique | [52] | |
| Centroceras sp. | Poisoned food technique | [52] | |
| Ceramium sp. | Poisoned food technique | [52] | |
| Chaetomorpha antennina | Poisoned food technique | [52] | |
| Codium indicum | Poisoned food technique | [52] | |
| Dictyopteris polypodioides | Agar diffusion technique | [63] | |
| Dictyota dicotoma | Poisoned food technique | [52] | |
| Gelidium pulchrum | Poisoned food technique | [52] | |
| Gracilaria corticata | Poisoned food technique | [52] | |
| Halimeda tuna | Poisoned food technique/Field studies | [52][71] | |
| Halymenia porphyriformis | Poisoned food technique | [52] | |
| Hormophysa cuneiformis | Agar diffusion assay/Broth microdilution assay | [56] | |
| Hypnea musciformis | Poisoned food technique | [52] | |
| Jania pedunculata var. adhaerens | Poisoned food technique | [52] | |
| Jolyna laminariodes | Poisoned food technique | [52] | |
| Melanothamnus afaqhusainii | Poisoned food technique/Field studies | [52][72] | |
| Neoporphyra perforata | Poisoned food technique | [52] | |
| Osmundea pinnatifida | Poisoned food technique | [52] | |
| Padina boergesenii | Disc diffusion technique | [119] | |
| Padina tetrastromatica | Poisoned food technique | [52][71] | |
| Polycladia indica | Poisoned food technique/Disc diffusion technique | [52][71][72][109] | |
| Polycladia myrica | Disc diffusion technique | [119] | |
| Sargassum aquifolium | Poisoned food technique | [52] | |
| Sargassum cinereum | Disc diffusion technique | [119] | |
| Sargassum ilicifolium | Disc diffusion technique | [109] | |
| Sargassum tenerrimum | Poisoned food technique | [52] | |
| Sargassum wightii | Poisoned food technique | [52] | |
| Scinaia huismanii | Poisoned food technique | [52] | |
| Spatoglossum asperum | Disc diffusion assay | [120] | |
| Steochospermum polypolides * | Poisoned food technique | [52] | |
| Udotea sp. | Poisoned food technique | [52] | |
| Ulva rigida | Poisoned food technique | [52] | |
| Valaniopsis sp. * | Poisoned food technique | [52] | |
| Fusarium oxysporum albedinis | Dictyota dichotoma | Disc diffusion technique | [65] |
| Dictyota implexa | Disc diffusion technique | [65] | |
| Dictyota spiralis | Disc diffusion technique | [65] | |
| Fusarium oxysporum dianthi | Dictyota dichotoma | Disc diffusion technique | [65] |
| Dictyota implexa | Disc diffusion technique | [65] | |
| Dictyota spiralis | Disc diffusion technique | [65] | |
| Fusarium oxysporum f.sp. udum | Caulerpa racemosa | Poisoned food technique | [105] |
| Hydropuntia edulis | Poisoned food technique | [105] | |
| Sargassum myricocystum * | Poisoned food technique | [105] | |
| Fusarium oxysporum lycopersici | Dictyota dichotoma | Disc diffusion technique | [65] |
| Dictyota implexa | Disc diffusion technique | [65] | |
| Dictyota spiralis | Disc diffusion technique | [65] | |
| Fusarium solani | Botryocladia leptopoda | Test tube in agar | [96] |
| Caulerpa racemosa | Test tube in agar | [96] | |
| Caulerpa taxifolia | Test tube in agar | [96] | |
| Champia compressa | Test tube in agar | [96] | |
| Codium indicum | Test tube in agar | [96] | |
| Gracilaria corticata | Test tube in agar | [96] | |
| Hypnea musciformis | Test tube in agar | [96] | |
| Hypnea valentiae | Test tube in agar | [96] | |
| Osmundea pinnatifida | Test tube in agar | [96] | |
| Padina antillarum | Test tube in agar | [96] | |
| Sarconema filiforme | Test tube in agar | [96] | |
| Sargassum ilicifolium | Test tube in agar | [96] | |
| Sargassum vulgare | Test tube in agar | [96][121] | |
| Solieria robusta | Test tube in agar/Field studies | [71][74][96][121] | |
| Spatoglossum asperum | Disc diffusion assay | [120] | |
| Stoechospermum polypodioides | Test tube in agar/Field studies | [71][74][96] | |
| Ulva lactuca | Test tube in agar | [96] | |
| Fusarium sp. | Anthophycus longifolius | Well diffusion technique | [51] |
| Ganoderma boninense | Caulerpa lamourouxii | Poisoned food technique | [122] |
| Caulerpa racemosa | Poisoned food technique | [122] | |
| Halimeda macrophysa | Poisoned food technique | [122] | |
| Sargassum oligocystum | Poisoned food technique | [122] | |
| Geotrichum sp. | Dictyopteris polypodioides | Agar diffusion technique | [63] |
| Macrophomina phaseolina | Calliblepharis floresii * | Poisoned food technique | [52] |
| Caulerpa racemosa | Poisoned food technique | [52] | |
| Caulerpa taxifolia | Poisoned food technique | [52] | |
| Centroceras sp. | Poisoned food technique | [52] | |
| Ceramium sp. | Poisoned food technique | [52] | |
| Chaetomorpha antennina | Poisoned food technique | [52] | |
| Codium indicum | Poisoned food technique | [52] | |
| Dictyota dicotoma | Poisoned food technique | [52] | |
| Gelidium pulchrum | Poisoned food technique | [52] | |
| Gracilaria corticata | Poisoned food technique | [52] | |
| Halymenia porphyriformis | Poisoned food technique | [52] | |
| Hypnea musciformis | Poisoned food technique | [52] | |
| Jania pedunculata var. adhaerens | Poisoned food technique | [52] | |
| Jolyna laminariodes | Poisoned food technique | [52] | |
| Melanothamnus afaqhusainii | Poisoned food technique | [52] | |
| Neoporphyra perforata | Poisoned food technique | [52] | |
| Osmundea pinnatifida | Poisoned food technique | [52] | |
| Padina tetrastromatica | Poisoned food technique | [52] | |
| Macrophomina phaseolina | Polycladia indica | Poisoned food technique/Disc diffusion technique | [52][109] |
| Sargassum aquifolium | Poisoned food technique | [52] | |
| Sargassum ilicifolium | Disc diffusion technique | [109] | |
| Sargassum tenerrimum | Poisoned food technique | [52] | |
| Sargassum wightii | Poisoned food technique | [52] | |
| Scinaia huismanii | Poisoned food technique | [52] | |
| Spatoglossum asperum | Disc diffusion assay | [120] | |
| Stoechospermum polypodioides | Poisoned food technique | [52] | |
| Udotea sp. | Poisoned food technique | [52] | |
| Ulva rigida | Poisoned food technique | [52] | |
| Valaniopsis sp. * | Poisoned food technique | [52] | |
| Mucor sp. | Champia compressa | Test tube in agar | [96] |
| Hypnea musciformis | Test tube in agar | [96] | |
| Sargassum boveanum | Test tube in agar | [96] | |
| Sargassum ilicifolium | Test tube in agar | [96] | |
| Ulva lactuca | Test tube in agar | [96] | |
| Penicillium expansum | Ulva lactuca | Disc diffusion technique | [58] |
| Penicillium sp. | Dictyota dichotoma | Disc diffusion technique | [123] |
| Ulva lactuca | Disc diffusion technique | [123] | |
| Penicillum digitatum | Hormophysa cuneiformis | Agar diffusion assay/Broth microdilution assay | [56] |
| Phialophora cinerescens | Dictyota dichotoma | Disc diffusion technique | [65] |
| Dictyota implexa | Disc diffusion technique | [65] | |
| Dictyota spiralis | Disc diffusion technique | [65] | |
| Phoma tracheiphila | Dictyota dichotoma | Disc diffusion technique | [65] |
| Dictyota implexa | Disc diffusion technique | [65] | |
| Dictyota spiralis | Disc diffusion technique | [65] | |
| Pseudocercospora fijiensis | Halymenia floresii | Minimum inhibitory concentration | [94] |
| Pyricularia oryzae | Rhodomela confervoides | Spore spreading method | [95] |
| Symphyocladia latiuscula | Spore spreading method | [95] | |
| Rhizoctonia solani | Calliblepharis floresii | Poisoned food technique | [52] |
| Centroceras sp. | Poisoned food technique | [52] | |
| Ceramium sp. | Poisoned food technique | [52] | |
| Chaetomorpha antennina | Poisoned food technique | [52] | |
| Codium indicum | Poisoned food technique | [52] | |
| Dictyopteris undulata | Fungitoxic activity | [92] | |
| Gelidium pulchrum | Poisoned food technique | [52] | |
| Gracilaria corticata | Poisoned food technique | [52] | |
| Halymenia porphyriformis | Poisoned food technique | [52] | |
| Hypnea musciformis | Poisoned food technique | [52] | |
| Jania pedunculata var. adhaerens | Poisoned food technique | [52] | |
| Melanothamnus afaqhusainii | Poisoned food technique | [52] | |
| Neoporphyra perforata | Poisoned food technique | [52] | |
| Osmundea pinnatifida | Poisoned food technique | [52] | |
| Padina tetrastromatica | Poisoned food technique | [52] | |
| Polycladia indica | Poisoned food technique | [52] | |
| Sargassum aquifolium | Poisoned food technique | [52][71][74] | |
| Sargassum tenerrimum | Poisoned food technique | [52][71] | |
| Rhizoctonia solani | Sargassum wightii | Poisoned food technique | [52] |
| Spatoglossum asperum | Disc diffusion assay/Field studies | [73][120] | |
| Stoechospermum polypodioides | Poisoned food technique/Field studies | [52][71][74] | |
| Udotea sp. | Poisoned food technique | [52] | |
| Ulva rigida | Poisoned food technique | [52] | |
| Valaniopsis sp. * | Poisoned food technique | [52] | |
| Dictyota dichotoma | Disc diffusion technique/Spore germination | [79] | |
| Padina gymnospora | Disc diffusion technique/Spore germination | [79] | |
| Sargassum muticum | Disc diffusion technique/Spore germination | [79] | |
| Sargassum tenerrimum | Disc diffusion technique/Spore germination | [79] | |
| Sargassum wightii | Disc diffusion technique/Spore germination | [79] | |
| Sclerotinia sclerotiorum | Dictyopteris undulata | Fungitoxic activity | [92] |
| Sclerotium rolfsii | Dictyopteris undulata | Fungitoxic activity | [92] |
| Verticillium dahliae | Cystoseira humilis var. myriophylloides | Poisoned food technique | [93] |
| Dictyopteris polypodioides | Agar diffusion technique | [63] | |
| Fucus spiralis | Poisoned food technique | [93] |
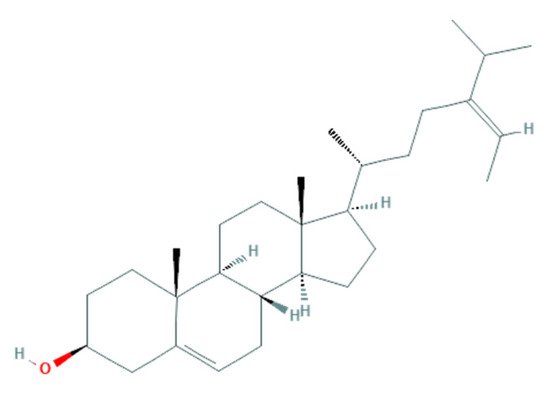
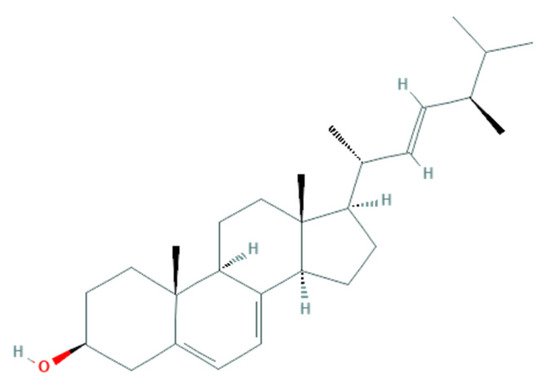
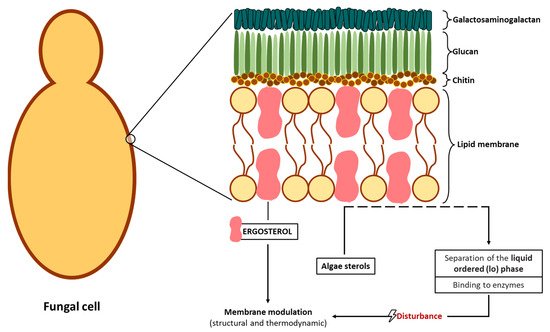
2.2.2. Potential Antifungal Mechanisms