Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vicky Zhou and Version 1 by Vicente Martínez.
Urinary tract infections (UTIs) represent a health problem of the first magnitude since they affect large segments of the population, cause increased mortality and comorbidity, and have a high incidence of relapse. Therefore, UTIs cause a major socioeconomic concern. Current antibiotic treatments have various limitations such as the appearance of resistance to antibiotics, nephrotoxicity, and side effects such as gastrointestinal problems including microbiota alterations that contribute to increasing antibiotic resistance. In this context, Itxasol© has emerged, approved as an adjuvant for the treatment of UTIs. Designed with biomimetic principles, it is composed of arbutin, umbelliferon, and N-acetyl cysteine.
- Itxasol©
- β-arbutin
- Urinary Tract Infections
1. Introduction
There are around 150 million urinary tract infections (UTIs) annually with a very high associated socioeconomic cost (in the USA alone, this is estimated to be in the order of $3.5 billion [1,2][1][2]). These infections affect women more than men, a fact that is related to the shorter length of the urethra in women than in men. Bacteria are capable of colonizing the urinary tract and moving up through it to reach the kidneys [3]. The pathology they can cause ranges from asymptomatic processes, to cystitis, and can become complicated even leading to cases of pyelonephritis [4,5][4][5]. As mentioned, these infections are more frequent in women and are associated with low socioeconomic levels related to poor hygiene in the menstrual period [6], sexual intercourse-postcoital UTIs [7], use of barrier contraceptive methods [8], and an increase with age related to alteration of hormone levels [9]. In the case of men, these infections are recurrent and are associated with cases of prostatitis, obstruction in the urethra, and benign hyperplasia [10]. Regardless of gender, the use of catheters is closely linked to the appearance of UTIs [11]. Reinfections in the case of UTIs are quite common although they vary according to age, with common occurrence of relapse after the first diagnosis [12].
One of the major problems associated with the use of antibiotics for the treatment of UTIs is the appearance of resistance to antibiotics that causes an increase in mortality, morbidity, and high socioeconomic costs. In addition to resistance to antibiotics, there are also collateral effects such as kidney damage, changes and alterations to the intestinal flora that cause digestive problems, and alterations to the metabolism and immunity [13,14,15,16][13][14][15][16].
The main bacteria associated with UTIs are Escherichia coli and Pseudomonas aueroginosa [17]. In the case of infections produced in hospitals, it has been shown that in addition to Escherichia coli and various enterobacteria, in this context, other bacteria such as Klebsiella spp. and Enterococcus spp. have been isolated from patients [18].
On the other hand, the formation of a biofilm by different bacterial strains has been related to an increase in resistance to antibiotics, as well as an increase in mortality and morbidity of diseases associated with infections caused by them [19,20][19][20]. The biofilm is an extracellular structure that is made up of sugars, lipid proteins, and molecules derived from DNA, which helps to spread bacteria and make them more resistant to antibiotics [21].
The most common antibiotic treatments for the management of UTIs are fosfomycin, nitrofurantoin, and quinolones to treat cystitis, and in the case of pyelonephritis, third-generation cephalosporins. In addition, in the case of administration for prophylactic purposes, trimethoprim + sulfamethoxazole, nitrofurantoin, cephalexin, and fosfomycin are used, while in the case of complicated cystitis, ciprofloxacin, levofloxacin, cefpodoxime, and ceftibuten are administered. In the case of having to administer a second line of treatments for pyelonephritis, cefepime, piperacillin/tazobactam, gentamycin, and amikacin are administered [22]. Table 1 describes the main antibiotics used against UTIs and their mechanisms of action.
Table 1.
Mechanisms of action of the various antibiotics.
| Antibiotic | Mechanism of Action | Reference |
|---|---|---|
| Nitrofurantoin | Destroys bacterial RNA and DNA | [23] |
| Fosfomycin | Inhibits Gram positive and negative cell wall synthesis | [23] |
| Ciprofloxacin | A fluoroquinolone used against Gram negative bacteria that impairs DNA’s bacterial synthesis and inhibits topoisomorases’ actions | [24] |
| Trimethoprim | Inhibits bacterial folic acid synthesis | [23] |
| Levofloxacin | Inhibits topoisomerase IV and bacterial gyrase | [25] |
| Cephalexin | Beta lactam that inhibits cell wall synthesis | [26] |
| Cefpodoxime | Cephalosporin that inhibits cell wall synthesis | [27] |
| Ceftibuten | Beta lactam that inhibits cell wall synthesis | [27] |
| Piperacillin | Beta lactam that inhibits cell wall synthesis | [27] |
Among the different causes generating resistance are their overuse, which enhances a favorable selective pressure when resistant strains spread and the ease with which resistance factors to treatments are transmitted between them. In addition, another aspect to take into account is bacterial resistance, understood as the ability of the system to return to the initial conditions after having suffered a disturbance to its state; in the case of bacteria, this implies temporary resistance to antibiotics that establishes a series of bacterial subpopulations. This concept is linked to genetic and non-genetic aspects that are manifested in three concepts: tolerance, resistance, and hetero-tolerance [28].
In particular, bacterial resistance can be associated, in the case of pathogens that cause UTIs, with the formation of biofilms. Biofilms are heterogeneous structures composed of bacteria and surrounded by a matrix [28]. These biofilms are related to the development of resistance to antibiotics, and it has been shown that the bacteria that produce these structures have a resistance to antibiotics that is 100 to 1000 times higher than that of bacteria that do not generate them.
Other causes of the appearance of resistance are to do with the characteristics of the host. It has been shown in an animal model that reinfections of UTIs are capable of altering the host cells, causing changes at the transcriptional level that affect the maturation of epithelial cells and that can remodel the composition of the epithelium, together with changes in inflammatory processes dependent on cyclooxygenase 2 [24].
The fact that certain pathogens can relatively easily infect the urinary tract is often related to their ability to adhere to the urinary epithelium through the presence of fimbriae or pilum, or to the capability of bacteria to adhere to each other by expressing adhesins, form biofilms, or generate molecules that can mask the natural response to lipopolysaccharide (LPS) [29,30][29][30]. Thus, the expression of certain genes by bacteria related to the production of fimbriae, biofilms, toxins, and adherence factors is related to the appearance of UTIs and to recurrent infections [31,32,33,34][31][32][33][34]. In addition, part of the damage produced by these bacteria is related to the inflammation processes caused in these infections, as in the case of pyelonephritis, a major complication of UTIs [35,36][35][36]. As we will demonstrate in this article, the mechanisms of action of the components of Itxasol© are directly related to these two important aspects: modulation of the expression of virulence genes, and regulation of inflammation.
2. Influence of Itxasol© in the Expression of Genes Related to Inflammation and Changes in Bacterial Genomics
2.1. β-Arbutin
Besides the described antimicrobial action, arbutin is also known for its anti-inflammatory action that modulates the genetic expression of different genes involved in this process. Table 2 summarizes the main actions of β-arbutin in this context.
Table 2.
Actions of β-arbutin.
| Action/Finding | References |
|---|
Table 3.
Actions of Umb.
| Action/Finding | Reference | |
|---|---|---|
| Reduces iNOS expression in B2 microglia cells and IL-1β, TNF-α, MCP-1, and IL-6 | [53] | [37] |
| Downregulation of genes involved in biofilm production and adhesion | [60] | [43] | |||
| Reduces oxidative stress levels in fibroblast and increases apoptosis of tumor cell line | [54] | [38] | |||
| Downregulation of genes related to production of extracellular matrix and motility | [61] | [44] | Increases expression of collagen I | [55] | [39] |
| Attenuation of DNA damage for oxidative stress | [62] | [45] | Decreases osteoclast activity | [56] | [40] |
| Reduction of inflammasome | [63] | [46] | No DNA damage in lymphocytes | [57,58] | [41][42] |
2.2. Umbelliferon (Umb)
In this section, we describe the main changes related to gene expression that Umb produces in bacteria and inflammation. Table 3 summarizes the major findings.
| Reduction of apoptosis of kidney cells | ||
| [ | ||
| 64 | ] | [47] |
| Produces DNA fragmentation in oral carcinoma cells | [65] | [48] |
| Cell cycle arrest in G1 apoptosis of adenocarcinoma cells | [66] | [49] |
2.3. N-acetyl-L-cysteine (NAC)
NAC can act against pathogens such as Escherichia coli and Enterococcus faecalis, preventing them from invading epithelial cells of the bladder [67][50]. This fact has been related to how the administration of NAC to cells can alter the synthesis of membrane proteins related to the recognition of bacteria in their first step, which is that of adhesion [68][51]. The actions of NAC in this context are summarized in Table 4.Table 4.
Actions of NAC.
| Action/Finding | References |
|---|
| Impairs adhesion of bacteria | [68] | [51] |
| Inhibits biofilm formation | [69,70,71] | [52][53][54] |
| DNA bacteria and biofilm components are derived from DNA destruction | [72,75] | [55][56] |
| Inhibits expression of genes related to fibrotic process | [74] | [57] |
| Reduces expression of genes related to apoptosis | [23] |
3. Conclusions and Future Directions
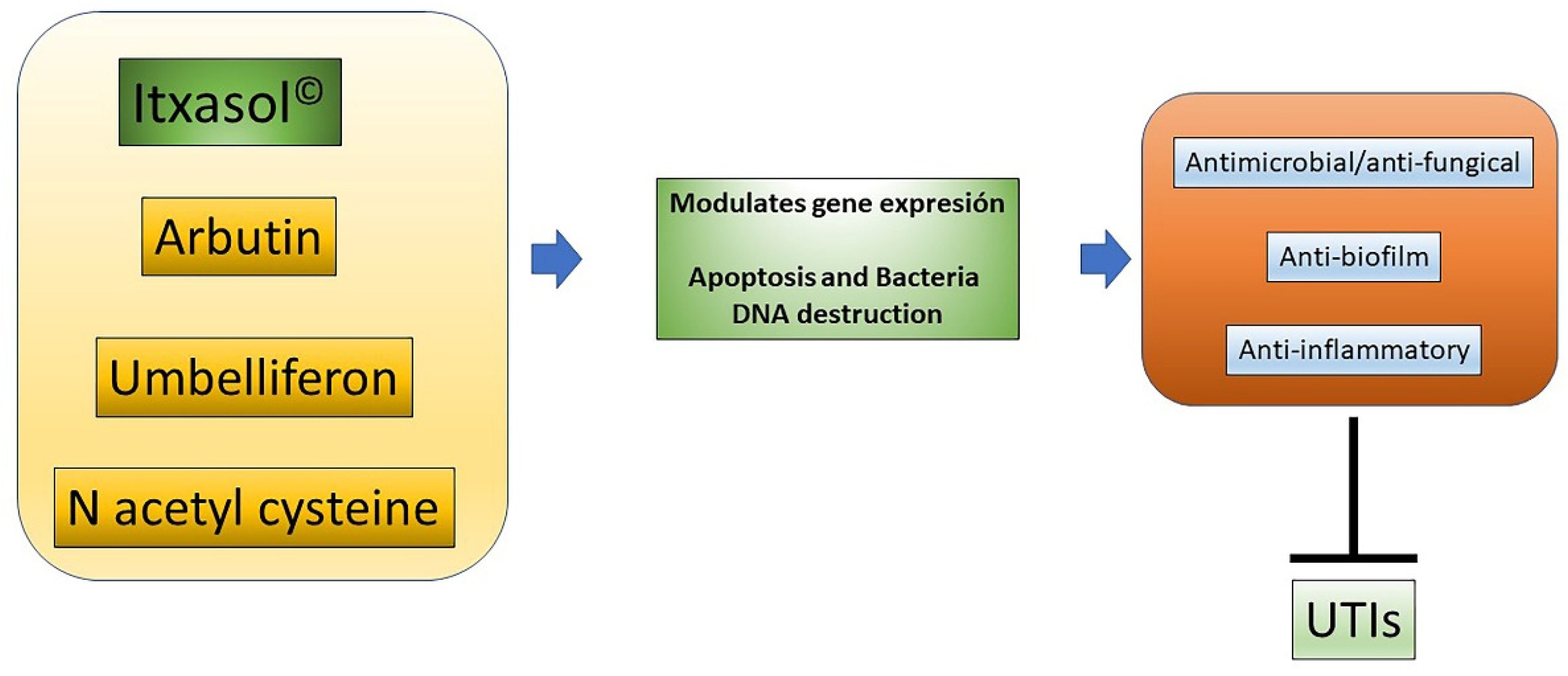
Given that UTIs have a high socioeconomic cost for society, there is a need to provide new ways to combat these infections. Itxasol© is a biomimetic compound that has antimicrobial, anti-biofilm, and anti-inflammatory characteristics that are very useful to fight this type of infection. As has been shown, these actions are carried out at least in part through the regulation of bacterial genes related to the expression of virulence factors and the formation of the biofilm, with modification of the expression patterns of genes related to inflammatory processes in different cell types, and with the modification of DNA cells to produce apoptosis (Figure 21). Considering the components of Itxasol©, Umb shows a clear capacity to interfere with the expression of genes related to adhesion and biofilm formation, β-arbutin′s main action is related to the regulation of inflammatory mechanisms, and NAC is able to destroy bacterial DNA and to control the inflammation produced by bacterial LPS.
Figure 21. Actions of Itxasol©. The different components of Itxasol© produce different changes in gene expression. To decrease the levels of inflammation in cells and bacteria, they interfere with the structure of DNA or the expression of virulence genes or those involved in the formation of biofilm. In this way, the combined anti-inflammatory and anti-biofilm antimicrobial actions can effectively combat UTIs.
At this point, it is necessary to carry out further studies to determine the genetic changes that both bacteria and host cells, including microbiota, have in the presence of the components of Itxasol©, both separately and in conjunction, i.e., resulting from this innovative formulation. As a result of these studies, it will be possible to improve the administration dose and guidelines for this alternative treatment adjuvant and perhaps to master a single administration where there is no need for antibiotics. In this way, it will be possible to improve the treatment of UTIs, reducing the possible adverse effects derived from traditional antibiotics in patients while simultaneously protecting the kidneys.
References
- Foxman, B. The Epidemiology of Urinary Tract Infection. Nat. Rev. Urol. 2010, 7, 653–660.
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284.
- Krzysztof, C.; Broś-Konopielko, M.; Teliga-Czajkowska, J. Urinary Tract Infection in Women. Prz. Menopauzalny 2021, 20, 40–47.
- Anger, J.T.; Saigal, C.S.; Wang, M.M.; Yano, E.M. Urologic Disease Burden in the United States: Veteran Users of Department of Veterans Affairs Healthcare. Urology 2008, 72, 37–41.
- Jennifer, A.; Lee, U.; Ackerman, A.L.; Chou, R.; Chughtai, B.; Clemens, J.Q.; Hickling, D.; Kapoor, A.; Kenton, K.S.; Kaufman, M.R.; et al. Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline. J. Urol. 2019, 202, 282–289.
- Mathiyalagen, P.; Peramasamy, B.; Vasudevan, K.; Basu, M.; Cherian, J.; Sundar, B. A Descriptive Cross-Sectional Study on Menstrual Hygiene and Perceived Reproductive Morbidity among Adolescent Girls in a Union Territory, India. J. Fam. Med. Prim. Care 2017, 6, 360.
- Nicolle, L.E.G.; Harding, K.M.; Preiksaitis, J.; Ronald, A.R. The Association of Urinary Tract Infection with Sexual Intercourse. J. Infect. Dis. 1982, 146, 579–583.
- Ingela, L.; Othman, J.; Hansson, M.; Ekelund, A.C.; Svanberg, T.; Strandell, A. New Types of Diaphragms and Cervical Caps versus Older Types of Diaphragms and Different Gels for Contraception: A Systematic Review. BMJ Sex. Reprod. Health 2020, 47, e12.
- Jung, C.; Brubaker, L. The Etiology and Management of Recurrent Urinary Tract Infections in Postmenopausal Women. Climacteric 2019, 22, 242–249.
- Manuel, E.; Galperine, T.; Caron, F. Urinary Tract Infections in Older Men. N. Engl. J. Med. 2016, 374, 2191–2192.
- Linsenmeyer, T.A. Catheter-Associated Urinary Tract Infections in Persons with Neurogenic Bladders. J. Spinal Cord Med. 2018, 41, 132–141.
- Betsy, F.; Gillespie, B.; Koopman, J.; Zhang, L.; Palin, K.; Tallman, P.; Marsh, J.V.; Spear, S.; Sobel, J.D.; Marty, M.J.; et al. Risk Factors for Second Urinary Tract Infection among College Women. Am. J. Epidemiol. 2000, 151, 1194–1205.
- Griebling, T.L. Urologic Diseases in America Project: Trends in Resource Use for Urinary Tract Infections in Women. J. Urol. 2005, 173, 1281–1287.
- Ventola, C.L. The Antibiotic Resistance Crisis: Causes and Threats. Pharm. Ther. J. 2015, 40, 277–283.
- Loukas, K.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415.
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37.
- Kambiz, A.; Fleischmann, N.; Schmiemann, G.; Bleidorn, J.; Hummers-Pradier, E.; Friede, T.; Wegscheider, K.; Moore, M.; Gágyor, I. Reducing Antibiotic Use for Uncomplicated Urinary Tract Infection in General Practice by Treatment with Uva-Ursi (REGATTA)—A Double-Blind, Randomized, Controlled Comparative Effectiveness Trial. BMC Complement. Altern. Med. 2018, 18, 203.
- Medina-Polo, J.; Naber, K.G.; Johansen, T.E.B. Healthcare-Associated Urinary Tract Infections in Urology. GMS Infect. Dis. 2021, 9, Doc05.
- Tanu, A.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, M.F.; Abid, M. Mechanistic Understanding of Candida albicans Biofilm Formation and Approaches for Its Inhibition. Front. Microbiol. 2021, 12, 932.
- Nikky, G.; Fatima, S.W.; Kumar, S.; Sinha, R.; Khare, S.K. Antimicrobial Resistance in Biofilms: Exploring Marine Actinobacteria as a Potential Source of Antibiotics and Biofilm Inhibitors. Biotechnol. Rep. 2021, 30, e00613.
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm Formation Mechanisms and Targets for Developing Antibiofilm Agents. Future Med. Chem. 2015, 7, 493–512.
- Goebel, M.C.; Trautner, B.W.; Grigoryan, L. The Five Ds of Outpatient Antibiotic Stewardship for Urinary Tract Infections. Clin. Microbiol. Rev. 2021, 34, e00003-20.
- Heng, F.; Le, J.W.; Zhu, J.H. Protective Effect of N-Acetylcysteine Pretreatment on Acute Kidney Injury in Septic Rats. J. Surg. Res. 2020, 254, 125–134.
- Yong, C.K.; Wong, S.K.; Ekeuku, S.O.; Pang, K.L. Relationship between Metabolic Syndrome and Bone Health—An Evaluation of Epidemiological Studies and Mechanisms Involved. Diabetes Metab. Syndr. Obes. Targets Ther. 2019.
- Hooper, D.C. Levofloxacin. In Kucers the Use of Antibiotics: A Clinical Review of Antibacterial Antifungal, Antiparasitic, and Antiviral Drugs, 7th ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 2055–2084.
- Nguyen, H.M.; Graber, C.J. A Critical Review of Cephalexin and Cefadroxil for the Treatment of Acute Uncomplicated Lower Urinary Tract Infection in the Era of ‘Bad Bugs, Few Drugs’. Int. J. Antimicrob. Agents 2020, 56, 106085.
- Davide, C.; Siracusa, C.; Sulejmani, A.; Leoni, V.; Intra, J. Old and New Beta-Lactamase Inhibitors: Molecular Structure, Mechanism of Action and Clinical Use. Antibiotics 2021, 10, 995.
- Gabriel, C.; Forestier, C.; Mathias, J.D. Antibiotic Resilience: A Necessary Concept to Complement Antibiotic Resistance? Proc. R. Soc. B Biol. Sci. 2019, 286, 20192408.
- Beerepoot, M.A.J.; Riet, G.T.; Nys, S.; Wal, W.M.V.D.; Corianne, A.J.M.D.B.; Reijke, T.M.D.; Prins, J.M.; Koeijers, J.; Verbon, A.; Stobberingh, E.; et al. Lactobacilli vs Antibiotics to Prevent Urinary Tract Infections: A Randomized, Double-Blind, Noninferiority Trial in Postmenopausal Women. Arch. Intern. Med. 2012, 172, 704–712.
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella Pneumoniae. Int. J. Environ. Res. Public Health 2020, 17, 6278.
- Vigil, P.D.; Stapleton, A.E.; Johnson, J.R.; Hooton, T.M.; Hodges, A.P.; He, Y.; Mobley, H.L.T. Presence of Putative Repeat-in-Toxin Gene TosA in Escherichia coli Predicts Successful Colonization of the Urinary Tract. MBio 2011, 2, e00066-11.
- Lüthje, P.; Brauner, A. Virulence Factors of Uropathogenic E. Coli and Their Interaction with the Host. Adv. Microb. Physiol. 2014, 65, 337–372.
- Sokurenko, E. Pathoadaptive Mutations in Uropathogenic Escherichia Coli. Microbiol. Spectr. 2016, 4, e01085-16.
- Lupo, F.; Ingersoll, M.A.; Pineda, M.A. The Glycobiology of Uropathogenic E. Coli Infection: The Sweet and Bitter Role of Sugars in Urinary Tract Immunity. Immunology 2021, 164, 3–14.
- Meyrier, A. Acute Pyelonephritis. Rev. Prat. 2003, 53, 1777–1784. Available online: https://pubmed.ncbi.nlm.nih.gov/14702820/ (accessed on 1 November 2021).
- Kranz, J.; Wagenlehner, F.M.E.; Schneidewind, L. Complicated Urinary Tract Infections. Urologe 2020, 59, 1480–1485.
- Jong, L.H.; Kim, K.W. Anti-Inflammatory Effects of Arbutin in Lipopolysaccharide-Stimulated BV2 Microglial Cells. Inflamm. Res. 2012, 61, 817–825.
- Shima, E.; Zabihi, E.; Golpour, M.; Aghajanpour-Mir, M. The Effect of Arbutin on the Expression of Tumor Suppressor P53, BAX/BCL-2 Ratio and Oxidative Stress Induced by Tert-Butyl Hydroperoxide in Fibroblast and LNcap Cell Lines. Cell J. 2021, 22, 532–541.
- Man, X.; Yang, L.; Liu, S.; Yang, L.; Li, M.; Fu, Q. Arbutin Promotes MC3T3-E1 Mouse Osteoblast Precursor Cell Proliferation and Differentiation via the Wnt/Β-catenin Signaling Pathway. Mol. Med. Rep. 2019, 19, 4637–4644.
- Akina, O.; Yoshimura, Y.; Deyama, Y.; Suzuki, K. Rosmarinic Acid and Arbutin Suppress Osteoclast Differentiation by Inhibiting Superoxide and NFATc1 Downregulation in RAW 264.7 Cells. Biomed. Rep. 2015, 3, 483–490.
- Jurica, K.; Karačonji, I.B.; Mikolić, A.; Milojković-Opsenica, D.; Benković, V.; Kopjar, N. In Vitro Safety Assessment of the Strawberry Tree (Arbutus unedo L.) Water Leaf Extract and Arbutin in Human Peripheral Blood Lymphocytes. Cytotechnology 2018, 70, 1261–1278.
- Jurica, K.; Benković, V.; Sikirić, S.; Karačonji, I.B.; Kopjar, N. The Effects of Strawberry Tree (Arbutus unedo L.) Water Leaf Extract and Arbutin upon Kidney Function and Primary DNA Damage in Renal Cells of Rats. Nat. Prod. Res. 2020, 34, 2354–2357.
- Chang, J.; Zhang, J.; Yu, L.; Wang, C.; Yang, Y.; Rong, X.; Xu, K.; Chu, M. Antifungal Activity of Coumarin against Candida albicans Is Related to Apoptosis. Front. Cell. Infect. Microbiol. 2019, 9, 445.
- Hyung, L.J.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins Reduce Biofilm Formation and the Virulence of Escherichia coli O157:H7. Phytomedicine 2014, 21, 1037–1042.
- Alotaibi, M.F.; Al-Joufi, F.; Seif, H.S.A.; Alzoghaibi, M.A.; Djouhri, L.; Ahmeda, A.F.; Mahmoud, A.M. Umbelliferone Inhibits Spermatogenic Defects and Testicular Injury in Lead-Intoxicated Rats by Suppressing Oxidative Stress and Inflammation, and Improving Nrf2/HO-1 Signaling. Drug Des. Dev. Ther. 2020, 14, 4003–4019.
- Hassanein, E.H.M.; Fares, E.M.A.; Kozman, M.R.; El-Ghafar, O.A.M.A. Umbelliferone Attenuates Gentamicin-Induced Renal Toxicity by Suppression of TLR-4/NF-ΚB-P65/NLRP-3 and JAK1/STAT-3 Signaling Pathways. Environ. Sci. Pollut. Res. 2021, 28, 11558–11571.
- Kim, H.-J.; Jin, B.-R.; An, H.-J. Umbelliferone Ameliorates Benign Prostatic Hyperplasia by Inhibiting Cell Proliferation and G1/S Phase Cell Cycle Progression through Regulation of STAT3/E2F1 Axis. Int. J. Mol. Sci. 2021, 22, 9019.
- Vijayalakshmi, A.; Sindhu, G. Umbelliferone Arrest Cell Cycle at G0/G1 Phase and Induces Apoptosis in Human Oral Carcinoma (KB) Cells Possibly via Oxidative DNA Damage. Biomed. Pharmacother. 2017, 92, 661–671.
- Lopez-Gonzalez, J.S.; Prado-Garcia, H.; Aguilar-Cazares, D.; Molina-Guarneros, J.A.; Morales-Fuentes, J.; Mandoki, J.J. Apoptosis and Cell Cycle Disturbances Induced by Coumarin and 7-Hydroxycoumarin on Human Lung Carcinoma Cell Lines. Lung Cancer 2004, 43, 275–283.
- Manoharan, A.; Ognenovska, S.; Paino, D.; Whiteley, G.; Glasbey, T.; Kriel, F.H.; Farrell, J.; Moore, K.H.; Manos, J.; Das, T. N-Acetylcysteine Protects Bladder Epithelial Cells from Bacterial Invasion and Displays Antibiofilm Activity against Urinary Tract Bacterial Pathogens. Antibiotics 2021, 10, 900.
- Irina, G.; Efremova, T.; Kirpichnikova, K.; Kever, L.; Komissarchik, Y.; Polozov, Y.; Khaitlina, S. N-Acetylcysteine-Induced Changes in Susceptibility of Transformed Eukaryotic Cells to Bacterial Invasion. Cell Biol. Int. 2006, 30, 319–325.
- Makipour, K.; Friedenberg, F.K. The Potential Role of N-Acetylcysteine for the Treatment of Helicobacter pylori. J. Clin. Gastroenterol. 2011, 45, 841–843.
- Zhao, T.; Liu, Y. N-Acetylcysteine Inhibit Biofilms Produced by Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 1–8.
- Arthika, M.; Das, T.; Whiteley, G.S.; Glasbey, T.; Kriel, F.H.; Manos, J. The Effect of N-Acetylcysteine in a Combined Antibiofilm Treatment against Antibiotic-Resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2020, 75, 1787–1798.
- Kundukad, B.; Schussman, M.; Yang, K.; Seviour, T.; Yang, L.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Mechanistic Action of Weak Acid Drugs on Biofilms. Sci. Rep. 2017, 7, 4783.
- Binu, K.; Udayakumar, G.; Grela, E.; Kaur, D.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Weak Acids as an Alternative Anti-Microbial Therapy. Biofilm 2020, 2, 100019.
- Ryu, C.M.; Shin, J.H.; Yu, H.Y.; Ju, H.; Kim, S.; Lim, J.; Heo, J.; Lee, S.; Shin, D.M.; Choo, M.S. N-Acetylcysteine Prevents Bladder Tissue Fibrosis in a Lipopolysaccharide-Induced Cystitis Rat Model. Sci. Rep. 2019, 9, 1–11.
More
