Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Yu-Kuan Huang.
Metastasis is considered one of the hallmarks of cancer and enhanced tumor invasion and metastasis is significantly associated with cancer mortality. Metastasis occurs via a series of integrated processes involving tumor cells and the tumor microenvironment. The innate immune components of the microenvironment have been shown to engage with tumor cells and not only regulate their proliferation and survival, but also modulate the surrounding environment to enable cancer progression.
- metastasis
- cancer
- immunity
- innate immunity
- invasion
1. Tumor Invasion and Metastasis
Metastasis is the general term used to describe the spread of cancer cells from the primary tumor to distant sites in the body and was reported by Hanahan and Weinberg as one of the hallmarks of cancer [1]. It has been reported that approximately 90% of cancer deaths are associated with metastases [2,3][2][3]. While metastasis has been considered to occur in later stages of disease progression, accumulation of earlier events during metastatic dissemination has been reported prior to clinically detectable tumors [4] and is one factor responsible for the concept of cancer with unknown primary.
The “metastatic cascade’’ describes the processes and restrictive bottlenecks through which tumor cells acquire cellular traits that allow them to exit primary tumor site and to travel and colonize distant organs. It involves major steps including local invasion, intravasation, and extravasation, which are well recognized in cancer [1,5,6][1][5][6] (Figure 1).
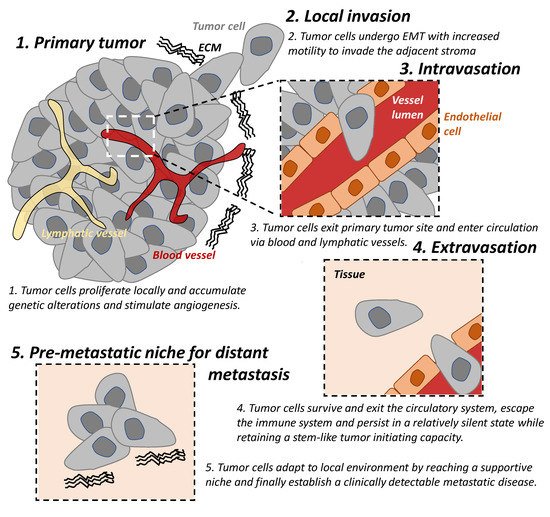
Figure 1. The tumor metastatic cascade. The “metastatic cascade” describes the processes and restrictive bottlenecks through which tumor cells acquire cellular traits, allowing them to exit the primary tumor site and migrate to distant organs which they then colonize. This occurs via a series of steps including local invasion, intravasation, and extravasation. EMT: epithelial-mesenchymal transition. ECM: extracellular matrix.
Local invasion occurs when tumor cells proliferate, accumulate genetic alterations, stimulate angiogenesis, and undergo epithelial-mesenchymal transition (EMT) locally. This result in the loss of cell-cell adhesion, degradation of the extracellular matrix (ECM), and basement membrane and allows for increased motility of the tumor cells to invade the adjacent stroma and is an important trait of malignancy [7,8][7][8].
In addition to the reliance on the blood supply of their host, tumor cells secrete angiogenic factors inducing abnormal vasculatures that are often leaky and immature [9]. These features result in tumor hypoxia which drives EMT of tumor cells and provide a route for mesenchymal cells to enter blood circulation; hence, there is a process of intravasation [9,10][9][10]. Lymphatic intravasation is an alternative pathway by which tumor cells can enter the circulation and metastasis to regional and distant lymph nodes [11].
The following step of the cascade is successful extravasation [5]. This requires tumor cells (A) to survive and exit the circulatory system, (B) to escape the immune system and persist in a relatively silent state while retaining a stem-like tumor initiating capacity, until (C) they adapt to local environment by reaching a supportive niche, and finally (D) establish a clinically detectable metastatic disease [5]. The majority of disseminated cells from primary tumors are eliminated by the immune system in circulation and in distant organs or remain dormant at metastatic sites and only very few develop into metastatic tumors [12,13][12][13]. Successful establishment of these metastatic tumors require tumor cells to metastasize to a favorable local microenvironment, as described by the “seed and soil” hypothesis [14].
2. Metastasis Modulated by Tumor Microenvironment and Immunity
While tumor cell-intrinsic factors including tumor heterogeneity are known to drive invasion and metastasis, extrinsic factors within the tumor microenvironment (TME) have been shown to play critical roles in determining the fate of cancer progression [15]. The stromal components of the TME are composed of several cell populations, including immune cells, fibroblasts, and endothelial cells [16]. The interaction between these cell types and the ECM, vascular distribution, oxygen (hypoxia), nutrients, and chemokines further complicates the tumor milieu [17].
The immune system has been demonstrated to shape the local TME [18] and contributes not only to the elimination of tumor cells, but may also be subverted to support their invasion and metastasis [19,20][19][20]. Under normal conditions, the Cancer-Immune cycle involving the dynamic communication of both innate and adaptive immune systems is activated to remove malignant cells in a process known as immune surveillance [21]. This cycle initiates as neo-antigens are taken up by antigen-presenting cells (APCs) of the innate immune system and presented to the adaptive immune system to acquire specific recognition of tumor cells. These activated T cells then migrate to the tumor site allowing them to target the tumor cells expressing these neo-antigens. Effector molecules such as interferon gamma (IFN-γ), tumor-necrosis factor alpha (TNF-α), as well as recognizing molecules (e.g., NKG2D) have been shown to be important in this process [22,23][22][23]. Functional immune surveillance results in the elimination of tumor cells; however, this is often disrupted or hijacked by cancer cells at multiple different points resulting in immune escape [21,24][21][24]. Possible routes include, but are not limited to, (A) downregulation or impairment of antigen presentation ability [25], (B) recruitment of pro-tumorigenic immune cells and secretion of inflammation-regulating cytokines by cells in the TME [26], and (C) upregulation of the expression of immune inhibitory molecules to suppress immune cell cytotoxicity [27]. Approaches that aim to restore an effective Cancer-Immune cycle are being investigated and are referred to as immune checkpoints [21].
Innate immunity (e.g., dendritic cells, macrophages, neutrophils, and natural killer cells) represents the first line of defense and rapid response to foreign substances and in anti-tumor activities [28]. However, these immune cells have also been shown to drive cancer-associated inflammation [1] and to participate in several steps of the metastatic cascade from reorganizing ECM [29], modulating vessel formation and permeability [9], and influencing cancer cell motility and states [30,31][30][31] (Figure 2). This remainder of this review summarizes known characteristics of innate immune cell populations on tumor invasion and metastasis and discusses recent advances in the field.
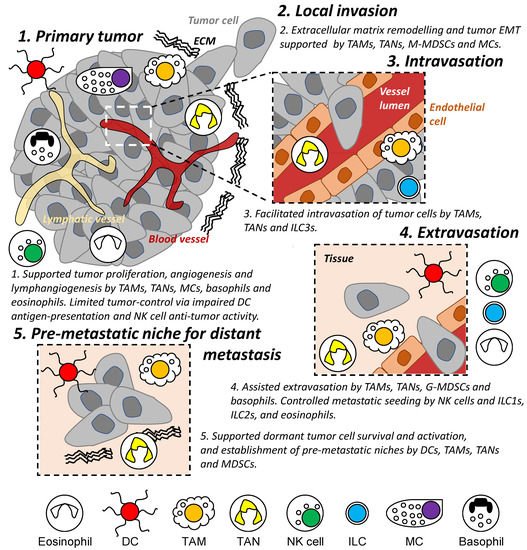
Figure 2. Role of innate immune cells during the metastatic cascade. Different populations of innate immune cells are involved in different stages of the metastatic cascade both locally and in distant organs/tissues, including regulating the survival and proliferation of tumor cells and assisting in the establishment of a permissive tissue environment enabling tumor cell invasion and migration. DC: dendritic cell. TAM: tumor-associated macrophage. TAN: tumor-associated neutrophil. M- and G-MDSC: monocytic and granulocytic-myeloid-derived suppressor cells. MC: mast cell. NK: natural killer. ILC: innate lymphoid cell. EMT: epithelial-mesenchymal transition. ECM: extracellular matrix.
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Bogenrieder, T.; Herlyn, M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncogene 2003, 22, 6524–6536.
- Blomberg, O.S.; Spagnuolo, L.; de Visser, K.E. Immune regulation of metastasis: Mechanistic insights and therapeutic opportunities. Dis. Models Mech. 2018, 11.
- Hosseini, H.; Obradovic, M.M.S.; Hoffmann, M.; Harper, K.L.; Sosa, M.S.; Werner-Klein, M.; Nanduri, L.K.; Werno, C.; Ehrl, C.; Maneck, M.; et al. Early dissemination seeds metastasis in breast cancer. Nature 2016, 540, 552–558.
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306.
- Pachmayr, E.; Treese, C.; Stein, U. Underlying Mechanisms for Distant Metastasis—Molecular Biology. Visc. Med. 2017, 33, 11–20.
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890.
- Beuran, M.; Negoi, I.; Paun, S.; Ion, A.D.; Bleotu, C.; Negoi, R.I.; Hostiuc, S. The epithelial to mesenchymal transition in pancreatic cancer: A systematic review. Pancreatology 2015, 15, 217–225.
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018, 9, 115.
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292.
- Paduch, R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell. Oncol. 2016, 39, 397–410.
- Strilic, B.; Offermanns, S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017, 32, 282–293.
- Faltas, B. Cornering metastases: Therapeutic targeting of circulating tumor cells and stem cells. Front. Oncol. 2012, 2, 68.
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A. Paget’s “Seed and Soil” Theory of Cancer Metastasis: An Idea Whose Time has Come. Adv. Anat. Pathol. 2019, 26, 69–74.
- D’Angelo, E.; Lindoso, R.S.; Sensi, F.; Pucciarelli, S.; Bussolati, B.; Agostini, M.; Collino, F. Intrinsic and Extrinsic Modulators of the Epithelial to Mesenchymal Transition: Driving the Fate of Tumor Microenvironment. Front. Oncol. 2020, 10, 1122.
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185.
- Yang, M.; McKay, D.; Pollard, J.W.; Lewis, C.E. Diverse Functions of Macrophages in Different Tumor Microenvironments. Cancer Res. 2018, 78, 5492–5503.
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022.
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437.
- Kather, J.N.; Halama, N. Harnessing the innate immune system and local immunological microenvironment to treat colorectal cancer. Br. J. Cancer 2019, 120, 871–882.
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10.
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011, 208, 1989–2003.
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271.
- Pio, R.; Ajona, D.; Ortiz-Espinosa, S.; Mantovani, A.; Lambris, J.D. Complementing the Cancer-Immunity Cycle. Front. Immunol. 2019, 10, 774.
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568.
- Wu, Y.; Zhou, B.P. Inflammation: A driving force speeds cancer metastasis. Cell Cycle 2009, 8, 3267–3273.
- Schnell, A.; Bod, L.; Madi, A.; Kuchroo, V.K. The yin and yang of co-inhibitory receptors: Toward anti-tumor immunity without autoimmunity. Cell Res. 2020, 30, 285–299.
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32.
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160.
- Stuelten, C.H.; Parent, C.A.; Montell, D.J. Cell motility in cancer invasion and metastasis: Insights from simple model organisms. Nat. Rev. Cancer 2018, 18, 296–312.
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86.
More
