Chronic inflammation is a major cause of human cancers. The environmental factors, such as microbiome, dietary components, and obesity, provoke chronic inflammation in the prostate, which promotes cancer development and progression. Crosstalk between immune cells and cancer cells enhances the secretion of intercellular signaling molecules, such as cytokines and chemokines, thereby orchestrating the generation of inflammatory microenvironment. Tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) play pivotal roles in inflammation-associated cancer by inhibiting effective anti-tumor immunity. Anti-inflammatory agents, such as aspirin, metformin, and statins, have potential application in chemoprevention of prostate cancer. Furthermore, pro-inflammatory immunity-targeted therapies may provide novel strategies to treat patients with cancer. Thus, anti-inflammatory agents are expected to suppress the “vicious cycle” created by immune and cancer cells and inhibit cancer progression. This review has explored the immune cells that facilitate prostate cancer development and progression, with particular focus on the application of anti-inflammatory agents for both chemoprevention and therapeutic approach in prostate cancer.
1. Introduction
Chronic inflammation plays a major role in the etiology and development of various types of malignant tumors, including hepatocellular carcinoma, gastric cancer, lung cancer, colorectal cancer, and prostate cancer [1][2][3][4][5]. Although inherited germline mutations are involved in prostate cancer development [6][7], immigration studies indicate the importance of environmental factors; for instance, it was found that immigrants from Asian countries in Western countries acquired higher prostate cancer risks within one generation [8][9]. The exposure to environmental factors, such as microbiome, cellular trauma, hormonal imbalances, dietary carcinogens, and obesity, leads to prostate epithelium injury and causes chronic inflammation [3][10][11][12]. In the adult prostate, chronic inflammation is prevalent and associated with putative precursor lesions that can provoke prostate cancer development [13][14][15][16].
Chronic inflammation plays a major role in the etiology and development of various types of malignant tumors, including hepatocellular carcinoma, gastric cancer, lung cancer, colorectal cancer, and prostate cancer [1,2,3,4,5]. Although inherited germline mutations are involved in prostate cancer development [6,7], immigration studies indicate the importance of environmental factors; for instance, it was found that immigrants from Asian countries in Western countries acquired higher prostate cancer risks within one generation [8,9]. The exposure to environmental factors, such as microbiome, cellular trauma, hormonal imbalances, dietary carcinogens, and obesity, leads to prostate epithelium injury and causes chronic inflammation [3,10,11,12]. In the adult prostate, chronic inflammation is prevalent and associated with putative precursor lesions that can provoke prostate cancer development [13,14,15,16].
A meta-analysis revealed an increased risk of prostate cancer among men with a history of prostatitis, syphilis, and gonorrhea [17]. Although a number of studies supported the idea of a connection between prostatitis and prostate cancer risk [18][19][20][21][22], subsequence studies revealed conflicting results [23][24][25][26][27]. The inconsistent results could be attributed to differences in the study population and potential selection bias, as acute and chronic prostatitis is associated with increased serum prostate-specific antigen (PSA) levels [28]. Previous epidemiological studies focused on the relationship between inflammation and prostate cancer development [29][30][31]. The first prospective study in men without biopsy indication revealed that benign tissue inflammation was positively associated with prostate cancer development [32]. Furthermore, the progression and aggressiveness of prostate cancer was reportedly associated with systemic inflammation markers in the serum, such as C-reactive protein levels, as well as differential blood cell count (neutrophils, lymphocytes, monocytes, and platelets) [33][34][35][36][37].
A meta-analysis revealed an increased risk of prostate cancer among men with a history of prostatitis, syphilis, and gonorrhea [17]. Although a number of studies supported the idea of a connection between prostatitis and prostate cancer risk [18,19,20,21,22], subsequence studies revealed conflicting results [23,24,25,26,27]. The inconsistent results could be attributed to differences in the study population and potential selection bias, as acute and chronic prostatitis is associated with increased serum prostate-specific antigen (PSA) levels [28]. Previous epidemiological studies focused on the relationship between inflammation and prostate cancer development [29,30,31]. The first prospective study in men without biopsy indication revealed that benign tissue inflammation was positively associated with prostate cancer development [32]. Furthermore, the progression and aggressiveness of prostate cancer was reportedly associated with systemic inflammation markers in the serum, such as C-reactive protein levels, as well as differential blood cell count (neutrophils, lymphocytes, monocytes, and platelets) [33,34,35,36,37].
The pathogenesis of inflammation-associated cancer is complex as both the innate and adaptive immune systems are involved in the process [38][39][40]. Chronic inflammation in the prostate microenvironment causes chronic increase in reactive oxygen species, which is associated with oxidative DNA damage in prostate epithelium [41]. Accumulated DNA damage can cause somatic mutations in key tumor suppressor genes and induce genome instability resulting in genomic changes in oncogenes, thus facilitating the development and progress of prostate cancer [3][42][43]. In fact, in patients with castration-resistant prostate cancer (CRPC), aberrations of
The pathogenesis of inflammation-associated cancer is complex as both the innate and adaptive immune systems are involved in the process [38,39,40]. Chronic inflammation in the prostate microenvironment causes chronic increase in reactive oxygen species, which is associated with oxidative DNA damage in prostate epithelium [41]. Accumulated DNA damage can cause somatic mutations in key tumor suppressor genes and induce genome instability resulting in genomic changes in oncogenes, thus facilitating the development and progress of prostate cancer [3,42,43]. In fact, in patients with castration-resistant prostate cancer (CRPC), aberrations of AR
(androgen receptor), E26 transformation-specific genes,
TP53
(tumor protein p53), and
PTEN (phosphatase and tensin homolog) were frequently observed (40–60% of cases), whereas only few (8%) cases had pathogenic germline alterations [44]. Additionally, inflammatory cells also secrete cytokines and chemokines that stimulate prostate cancer growth, angiogenesis, invasion, and metastasis [45][46][47]. Thus, anti-inflammatory agents are expected to suppress inflammation in the tumor microenvironment and inhibit prostate cancer progression (
(phosphatase and tensin homolog) were frequently observed (40–60% of cases), whereas only few (8%) cases had pathogenic germline alterations [44]. Additionally, inflammatory cells also secrete cytokines and chemokines that stimulate prostate cancer growth, angiogenesis, invasion, and metastasis [45,46,47]. Thus, anti-inflammatory agents are expected to suppress inflammation in the tumor microenvironment and inhibit prostate cancer progression ( ).
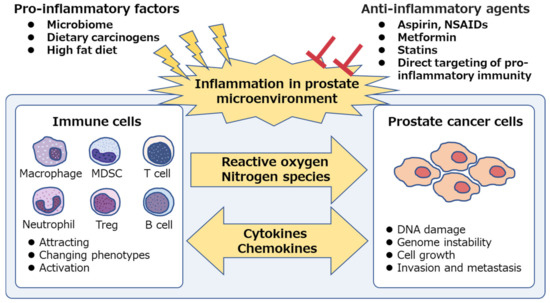
Figure 1.
Chronic inflammation is associated with prostate cancer. Pro-inflammatory factors, such as microbiome and dietary components, are the potential cause of prostatic inflammation. Immune cells secrete reactive oxygen and nitrogen species, induce DNA damage and genome instability in prostate epithelium and cause prostate cancer development. Both immune cells and prostate cancer cells secrete intercellular signaling molecules, such as cytokines and chemokines, and contribute to the generation of inflammatory microenvironment, which facilitates cancer progression. Anti-inflammatory agents suppress the “vicious cycle” and inhibit prostate cancer development and progression. NSAIDs, non-steroidal anti-inflammatory drugs; MDSC, myeloid-derived suppressor cell; Treg, regulatory T cell.
2. Immune Cells Involved in Inflammation and Prostate Cancer Progression
Cancer development and its response to therapy are strongly affected by innate and adaptive immunity, which either promote or attenuate tumorigenesis and can have opposing effects on therapeutic outcome [48]. A large number of studies have focused on immune cells in prostate cancer, including innate immune cells: Macrophages, neutrophils, and mast cells; adaptive immune cells: T cells and B cells; and immune-suppressive cells: regulatory T cells (Treg cells) and myeloid-derived suppressor cells (MDSCs) [49]. In the prostate microenvironment, these immune cells act as either friends or foes [50]. Growing evidence suggests that both macrophages and MDSCs play pivotal roles in inflammation-associated prostate cancer development and progression via down-regulation of effective anti-tumor immunity ( ). Although the precise molecular mechanisms involved are still unclear, animal models of prostate cancer, which mimic the human disease, have contributed to the determination of specific pathways and helped develop novel therapeutic agents.
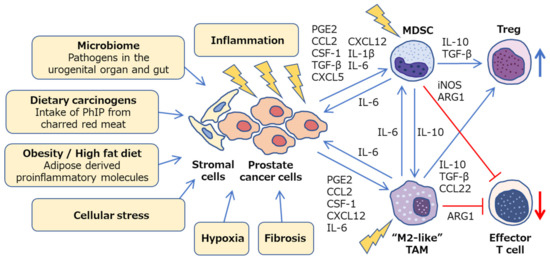
Figure 2.
Inflammatory microenvironment in prostate cancer. Diverse mechanisms, such as microbiome, dietary carcinogens, obesity, cellular stress, hypoxia and fibrosis, can be a potential cause of inflammation in prostate cancer. Crosstalk between cancer cells, stromal cells, and immune cells promotes chronic inflammation and facilitates prostate cancer progression via a variety of intercellular signaling molecules. Prostate tumor microenvironment is generally considered to be immunologically “cold” as M2-like TAMs and MDSCs cooperatively inhibit effector T cells and activate regulatory T cells. PhIP, 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine; PGE2, prostaglandin E2; CSF-1, colony stimulating factor-1; TGF-β
, transforming growth factor-β
; ARG1, arginase 1; iNOS, inducible nitric oxide synthase; TAM, tumor-associated macrophage; MDSC, myeloid-derived suppressor cell; Treg, regulatory T cell.
2.1. Macrophages
Tumor-associated macrophages (TAMs) are crucial drivers of tumor promoting inflammation and are generally associated with poor prognosis in solid tumors [51], including prostate cancer [52][53][54][55][56][57][58]. TAMs contribute to tumor progression at different levels by promoting genetic instability, cell proliferation, angiogenesis, and metastasis, as well as suppressing protective adaptive immunity [59][60][61][62][63]. Historically, macrophages are divided into classically activated macrophages (M1) and alternatively activated macrophages (M2), exhibiting anti-tumoral and pro-tumoral properties, respectively. Although interleukin (IL)-4 and IL-13 are the acknowledged signals that regulate M2 polarization of macrophages, recently other subtypes of the M2 class have been identified [64][65][66][67]. Thus, TAM population is referred to as “M2-like” when they include diverse phenotypes that share the functional outputs of tumor promotion and adaptive immunity suppression; these typically express characteristic surface molecules, such as CD163, CD204, and CD206 [62].
Tumor-associated macrophages (TAMs) are crucial drivers of tumor promoting inflammation and are generally associated with poor prognosis in solid tumors [51], including prostate cancer [52,53,54,55,56,57,58]. TAMs contribute to tumor progression at different levels by promoting genetic instability, cell proliferation, angiogenesis, and metastasis, as well as suppressing protective adaptive immunity [59,60,61,62,63]. Historically, macrophages are divided into classically activated macrophages (M1) and alternatively activated macrophages (M2), exhibiting anti-tumoral and pro-tumoral properties, respectively. Although interleukin (IL)-4 and IL-13 are the acknowledged signals that regulate M2 polarization of macrophages, recently other subtypes of the M2 class have been identified [64,65,66,67]. Thus, TAM population is referred to as “M2-like” when they include diverse phenotypes that share the functional outputs of tumor promotion and adaptive immunity suppression; these typically express characteristic surface molecules, such as CD163, CD204, and CD206 [62].
Furthermore, elements within the tumor microenvironment, such as hypoxia, fibrosis, cellular stress, and inflammation, dramatically shift the macrophage polarity towards M2-like phenotypes [68][69][70] (
Furthermore, elements within the tumor microenvironment, such as hypoxia, fibrosis, cellular stress, and inflammation, dramatically shift the macrophage polarity towards M2-like phenotypes [68,69,70] ( ). Many studies have shed light on the complex signaling network that drives myeloid cells toward M2-like TAMs, which involves various cytokines, chemokines, and signals within the tumor microenvironment [71], including prostaglandin E2 (PGE2) [72], chemokine (C–C motif) ligand (CCL)2 [73], colony stimulating factor (CSF)-1 [74], C–X–C motif chemokine (CXCL)12, and IL-6 [75][76]. Thus, tumor microenvironment can influence TAM polarization by releasing various factors that give rise to a large spectrum of pro-tumoral TAMs [77]. TAMs inhibit effector T cells by secreting IL-10, transforming growth factor (TGF)-
). Many studies have shed light on the complex signaling network that drives myeloid cells toward M2-like TAMs, which involves various cytokines, chemokines, and signals within the tumor microenvironment [71], including prostaglandin E2 (PGE2) [72], chemokine (C–C motif) ligand (CCL)2 [73], colony stimulating factor (CSF)-1 [74], C–X–C motif chemokine (CXCL)12, and IL-6 [75,76]. Thus, tumor microenvironment can influence TAM polarization by releasing various factors that give rise to a large spectrum of pro-tumoral TAMs [77]. TAMs inhibit effector T cells by secreting IL-10, transforming growth factor (TGF)- β
, and arginase (ARG)1 as well as via direct cell to cell contact. They also induce Treg cells via IL-10 and TGF-
β [78][79] (
). Therefore, TAMs can be potential therapeutic targets, and re-education of pro-tumoral M2-like TAMs toward anti-tumorigenic phenotype may be a potent strategy to treat prostate cancer [80][81].
). Therefore, TAMs can be potential therapeutic targets, and re-education of pro-tumoral M2-like TAMs toward anti-tumorigenic phenotype may be a potent strategy to treat prostate cancer [80,81].
2.2. MDSCs
MDSCs are a tolerogenic and immune-suppressive population of myeloid cells that are significantly expanded in patients with various types of cancers. High MDSC number negatively correlates with disease progression and overall survival, thus suggesting these to be a possible target for cancer immunotherapy [82][83]. MDSCs have distinct phenotypic surface markers as well as functional characteristics, particularly T cell activity inhibition. MDSCs were originally identified in mice as Gr-1
MDSCs are a tolerogenic and immune-suppressive population of myeloid cells that are significantly expanded in patients with various types of cancers. High MDSC number negatively correlates with disease progression and overall survival, thus suggesting these to be a possible target for cancer immunotherapy [82,83]. MDSCs have distinct phenotypic surface markers as well as functional characteristics, particularly T cell activity inhibition. MDSCs were originally identified in mice as Gr-1 +
CD11b
+ cells [84][85]. The Gr-1 marker is not a singular molecule, but a combination of Ly6C and Ly6G markers. Currently, MDSCs include two major subsets based on their phenotypic and morphological features: Polymorphonuclear (PMN)-MDSCs, including CD11b
cells [84,85]. The Gr-1 marker is not a singular molecule, but a combination of Ly6C and Ly6G markers. Currently, MDSCs include two major subsets based on their phenotypic and morphological features: Polymorphonuclear (PMN)-MDSCs, including CD11b +
Ly6C
lo
Ly6G
+
cells, and monocytic (M)-MDSCs, including CD11b
+
Ly6C
hi
Ly6G
−
cells. In humans, PMN-MDSC equivalent cells are defined as CD11b
+
CD14
−
CD15
+
or CD11b
+
CD14
−
CD66b
+
and M-MDSCs as CD11b
+
CD14
+
HLA-DR
low/−
CD15
− [86]. Growing evidence suggests that MDSCs play an important role in cancer development and progression via suppression of anti-tumoral T cell function in patients with prostate cancer [87][88][89][90][91][92]. However, the MDSC subsets that have clinical relevance during disease progression remain to be identified.
[86]. Growing evidence suggests that MDSCs play an important role in cancer development and progression via suppression of anti-tumoral T cell function in patients with prostate cancer [87,88,89,90,91,92]. However, the MDSC subsets that have clinical relevance during disease progression remain to be identified.
To date, various signaling pathways, including PGE2 [93], CCL2 [94][95], CSF-1 [96], TGF-
To date, various signaling pathways, including PGE2 [93], CCL2 [94,95], CSF-1 [96], TGF- β
[97], CXCL5, CXCL12 [98], IL-1 β [99][100], and IL-6 [101], have been identified to be involved in the infiltration and activation of MDSCs in tumor microenvironment [102][103][104][105][106]. MDSCs suppress anti-tumor immunity through a variety of diverse mechanisms, including ARG1, inducible nitric oxide synthase (iNOS), TGF-
[99,100], and IL-6 [101], have been identified to be involved in the infiltration and activation of MDSCs in tumor microenvironment [102,103,104,105,106]. MDSCs suppress anti-tumor immunity through a variety of diverse mechanisms, including ARG1, inducible nitric oxide synthase (iNOS), TGF- β, and IL-10, and PMN-MDSCs and M-MDSCs exhibit different mechanisms of immune suppression [107][108][109][110][111][112]. Ultimately, MDSCs inhibit the activation and clonal expansion of tumor-specific T cells, as well as induce Treg cell development (
, and IL-10, and PMN-MDSCs and M-MDSCs exhibit different mechanisms of immune suppression [107,108,109,110,111,112]. Ultimately, MDSCs inhibit the activation and clonal expansion of tumor-specific T cells, as well as induce Treg cell development ( ). Furthermore, MDSCs secrete various factors that promote prostate cancer progression, such as IL-1 receptor antagonist (IL-1RA) that antagonizes senescence of prostate cancer in a paracrine manner [113], and IL-23 that acts as a driver of CRPC [114]. Thus, targeting MDSCs may provide novel opportunities for cancer therapy.
). Furthermore, MDSCs secrete various factors that promote prostate cancer progression, such as IL-1 receptor antagonist (IL-1RA) that antagonizes senescence of prostate cancer in a paracrine manner [113], and IL-23 that acts as a driver of CRPC [114]. Thus, targeting MDSCs may provide novel opportunities for cancer therapy.
2.3. Crosstalk between Immune Cells, Stromal Cells, and Cancer Cells in Prostate Microenvironment
In the inflammatory prostate microenvironment, crosstalk between immune, stromal, and cancer cells potentially facilitates further tumor progression [115][116][117]. An ex vivo prostate tumor model, derived from patients with prostate cancer, demonstrated that prostate tumors showed low levels of cytotoxic T lymphocytes and T-helper (Th)1 cells-recruiting chemokines, such as CCL5, CXCL9, and CXCL10, but expressed high levels of chemokines implicated in attracting TAMs, MDSCs, and Treg cells, such as CCL2, CCL22, and CXCL12 [118]. CCL22, secreted by tumor cells and TAMs, activates trafficking of Treg cells within the tumor as well as promotes tumor migration and invasion [119][120][121]. Human prostate carcinoma-associated fibroblasts and prostate cancer cells orchestrate and enhance TAM and MDSC recruitment to prostate tumors as well as M2-like TAM differentiation by the chemokines CCL2, CXCL12, and IL-6 during cancer progression [76][122]. Sexually transmitted disease-associated inflammation facilitates IL-6 production by prostate epithelial cells, which induces M2-like TAM polarization [123]. Furthermore, MDSC and tumor cell cross-talk enhances IL-6 production within tumor microenvironment [124], while IL-10, produced by MDSCs, increases M2-like TAMs [125][126][127]. The MDSCs derived from patients with prostate cancer inhibit CD8
In the inflammatory prostate microenvironment, crosstalk between immune, stromal, and cancer cells potentially facilitates further tumor progression [115,116,117]. An ex vivo prostate tumor model, derived from patients with prostate cancer, demonstrated that prostate tumors showed low levels of cytotoxic T lymphocytes and T-helper (Th)1 cells-recruiting chemokines, such as CCL5, CXCL9, and CXCL10, but expressed high levels of chemokines implicated in attracting TAMs, MDSCs, and Treg cells, such as CCL2, CCL22, and CXCL12 [118]. CCL22, secreted by tumor cells and TAMs, activates trafficking of Treg cells within the tumor as well as promotes tumor migration and invasion [119,120,121]. Human prostate carcinoma-associated fibroblasts and prostate cancer cells orchestrate and enhance TAM and MDSC recruitment to prostate tumors as well as M2-like TAM differentiation by the chemokines CCL2, CXCL12, and IL-6 during cancer progression [76,122]. Sexually transmitted disease-associated inflammation facilitates IL-6 production by prostate epithelial cells, which induces M2-like TAM polarization [123]. Furthermore, MDSC and tumor cell cross-talk enhances IL-6 production within tumor microenvironment [124], while IL-10, produced by MDSCs, increases M2-like TAMs [125,126,127]. The MDSCs derived from patients with prostate cancer inhibit CD8 + T cells through ARG1, a downstream signal transducer and activator of transcription (STAT)3 target gene [91]. Moreover, the phenotypic analysis of prostate infiltrating lymphocytes, derived from patients with prostate cancer, revealed them to be skewed towards a regulatory Treg and Th17 phenotypes [128]. Tregs are associated with poor prognosis and were found to be highly infiltrated in the prostate tissue of patients with prostate cancer [129][130]. Th17 cells, the key mediators in a number of autoimmune diseases, play a role in inflammation-associated prostate cancer [131][132]. Their development depends on the pleiotropic cytokine TGF-
T cells through ARG1, a downstream signal transducer and activator of transcription (STAT)3 target gene [91]. Moreover, the phenotypic analysis of prostate infiltrating lymphocytes, derived from patients with prostate cancer, revealed them to be skewed towards a regulatory Treg and Th17 phenotypes [128]. Tregs are associated with poor prognosis and were found to be highly infiltrated in the prostate tissue of patients with prostate cancer [129,130]. Th17 cells, the key mediators in a number of autoimmune diseases, play a role in inflammation-associated prostate cancer [131,132]. Their development depends on the pleiotropic cytokine TGF- β
, which is also linked to Treg cell development and function [133]. In Hi-Myc mouse model of prostate cancer, retrograde urethral instillation of CP1, a human prostatic isolate of Escherichia coli,
was reported to induce chronic inflammation characterized by an influx of TAMs and Th17 lymphocytes with distinct cytokine profiles and thus accelerate cancer progression [134].
Crosstalk between MDSCs and mast cells was found to further suppress effective anti-tumor immunity in a transgenic adenocarcinoma of the mouse prostate (TRAMP) model [135]. PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine), one of the most abundant heterocyclic amines in cooked meat, induced rat prostate cancer with elevated DNA mutation frequencies in the prostate as well as infiltration of TAMs and mast cells, suggesting a potential mechanism involving inflammation promotion by which dietary compounds can increase cancer risk [136]. Furthermore, bacterial prostatitis accelerates PhIP-induced prostate carcinogenesis by increasing the level of circulating IL-6 in the rat prostate [137]. Another factor that facilitates chronic inflammation is obesity, wherein adipose derived proinflammatory molecules activate TAMs and MDSCs, subsequently promoting cancer progression [138][139][140][141][142][143][144][145]. CCL2 produced by adipocytes enhances the growth and invasion of prostate cancer cells [146]. Upregulation of serum CCL2 levels enhanced the tumor growth of prostate cancer LNCaP xenografts in high-fat diet fed mice [147]. In obese mice, expanded MDSCs suppress CD8
Crosstalk between MDSCs and mast cells was found to further suppress effective anti-tumor immunity in a transgenic adenocarcinoma of the mouse prostate (TRAMP) model [135]. PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine), one of the most abundant heterocyclic amines in cooked meat, induced rat prostate cancer with elevated DNA mutation frequencies in the prostate as well as infiltration of TAMs and mast cells, suggesting a potential mechanism involving inflammation promotion by which dietary compounds can increase cancer risk [136]. Furthermore, bacterial prostatitis accelerates PhIP-induced prostate carcinogenesis by increasing the level of circulating IL-6 in the rat prostate [137]. Another factor that facilitates chronic inflammation is obesity, wherein adipose derived proinflammatory molecules activate TAMs and MDSCs, subsequently promoting cancer progression [138,139,140,141,142,143,144,145]. CCL2 produced by adipocytes enhances the growth and invasion of prostate cancer cells [146]. Upregulation of serum CCL2 levels enhanced the tumor growth of prostate cancer LNCaP xenografts in high-fat diet fed mice [147]. In obese mice, expanded MDSCs suppress CD8 +
T cells via iNOS and interferon-
γ, and also induce M2 TAM polarization via IL-10 [148][149]. The loss of
, and also induce M2 TAM polarization via IL-10 [148,149]. The loss of Pten
in the prostate epithelium causes local MDSC expansion via inflammatory cytokines, such as CSF-1 and IL-1
β
. [
96
]. In a mouse prostate cancer model driven by loss of
Pten
and
Smad4
, MDSCs play a critical role in cancer progression, as CXCR2-expressing MDSCs infiltrate in the prostate due to CXCL5 up-regulation in tumors [150]. In Pten
-deficient model mice for prostate cancer, a high-fat diet mediated inflammation-induced M2 TAM differentiation and expansion of MDSCs, accelerated IL-6 secretion, and facilitated tumor growth via IL-6/STAT3 signaling pathway [151]. Thus, the inflammation-associated prostate cancer progression is potentially mediated by diverse mechanisms, such as microbiome, dietary carcinogens, obesity, cellular stress, hypoxia, and fibrosis, which consequently inhibit effective anti-tumor immunity ( ). These pathways may be promising targets for chemoprevention and cancer therapy.