Diabetes mellitus (DM), one of the metabolic diseases which is characterized by sustained hyperglycemia, is a life-threatening disease. The global prevalence of DM is on the rise, mainly in low- and middle-income countries. Diabetes is a major cause of blindness, heart attacks, kidney failure, stroke, and lower limb amputation. Type 2 diabetes mellitus (T2DM) is a form of diabetes that is characterized by high blood sugar and insulin resistance. T2DM can be prevented or delayed by a healthy diet, regular physical activity, maintaining normal body weight, and avoiding alcohol and tobacco use. Ethanol and its metabolites can cause differentiation defects in stem cells and promote inflammatory injury and carcinogenesis in several tissues. Studies have suggested that diabetes can be treated, and its consequences can be avoided or delayed with proper management. DM has a greater risk for several cancers, such as breast, colorectal, endometrial, pancreatic, gallbladder, renal, and liver cancer. The incidence of cancer is significantly higher in patients with DM than in those without DM. In addition to DM, alcohol abuse is also a risk factor for many cancers.
- diabetes mellitus
- alcoholism
- breast cancer
- pancreatic cancers
- gastric cancer
- colorectal cancer
- bladder cancer
1. Introduction
The possible biological links between diabetes mellitus or impaired glucose tolerance and cancer comprise hyperinsulinemia, hyperglycemia, and fat-induced chronic inflammation. DM is a known risk factor for several cancers [1], resulting from insulin resistance induced by a paraneoplastic syndrome [2] or pancreatic β-cell dysfunction [3]. Mechanistically, hyperglycemia may cause hyperinsulinemia, providing growth signals to positively stimulate the expansion of cancer [4,5,6][4][5][6]. In addition, it has been demonstrated that moderate alcohol intake had no significant impact, whereas high alcohol intake was associated with an increased risk of breast and gastrointestinal cancer [7,8,9,10][7][8][9][10].
According to the National Diabetes Statistics Report, a periodical publication by the Centers for Disease Control and Prevention (CDC), during 1999–2016, the age-adjusted prevalence of total diabetes significantly increased among adults aged 18 years or older. Prevalence estimates were 9.5% in 1999–2002 and 12.0% in 2013–2016. Among the overall US population, the crude estimates for 2018 were that 34.2 million people of all ages or 10.5% of the US population had diabetes. Furthermore, 34.1 million adults aged 18 years or older, or 13.0% of all US adults, had diabetes. Age-adjusted data for 2017–2018 indicated that non-Hispanic blacks (8.2 per 1000 persons) and people of Hispanic origin (9.7 per 1000 persons) had a higher incidence of diabetes compared to non-Hispanic whites (5.0 per 1000 persons). According to the National Institute of Diabetes and Digestive and Kidney Diseases, diabetes is the seventh leading cause of death in the United States.
In 2017, the International Agency for Research on Cancer (IARC) concluded that obesity is a risk factor of cancer of 13 anatomic sites [11]. The direct association of diabetes mellitus with pancreatic, liver, breast, endometrium, bladder, and kidney cancer has been demonstrated. In addition to obesity and diabetes, other risk factors of cancer are alcohol abuse, genetics (family history), smoking, and exposure to toxic chemicals ( Figure 1 ). Recent studies have shown an association between the incidence of cancer and anti-diabetic medications. Furthermore, the use of metformin (a drug for type 2 diabetes mellitus) is associated with a reduced risk of cancer [12,13,14,15,16][12][13][14][15][16] or cancer mortality [17].
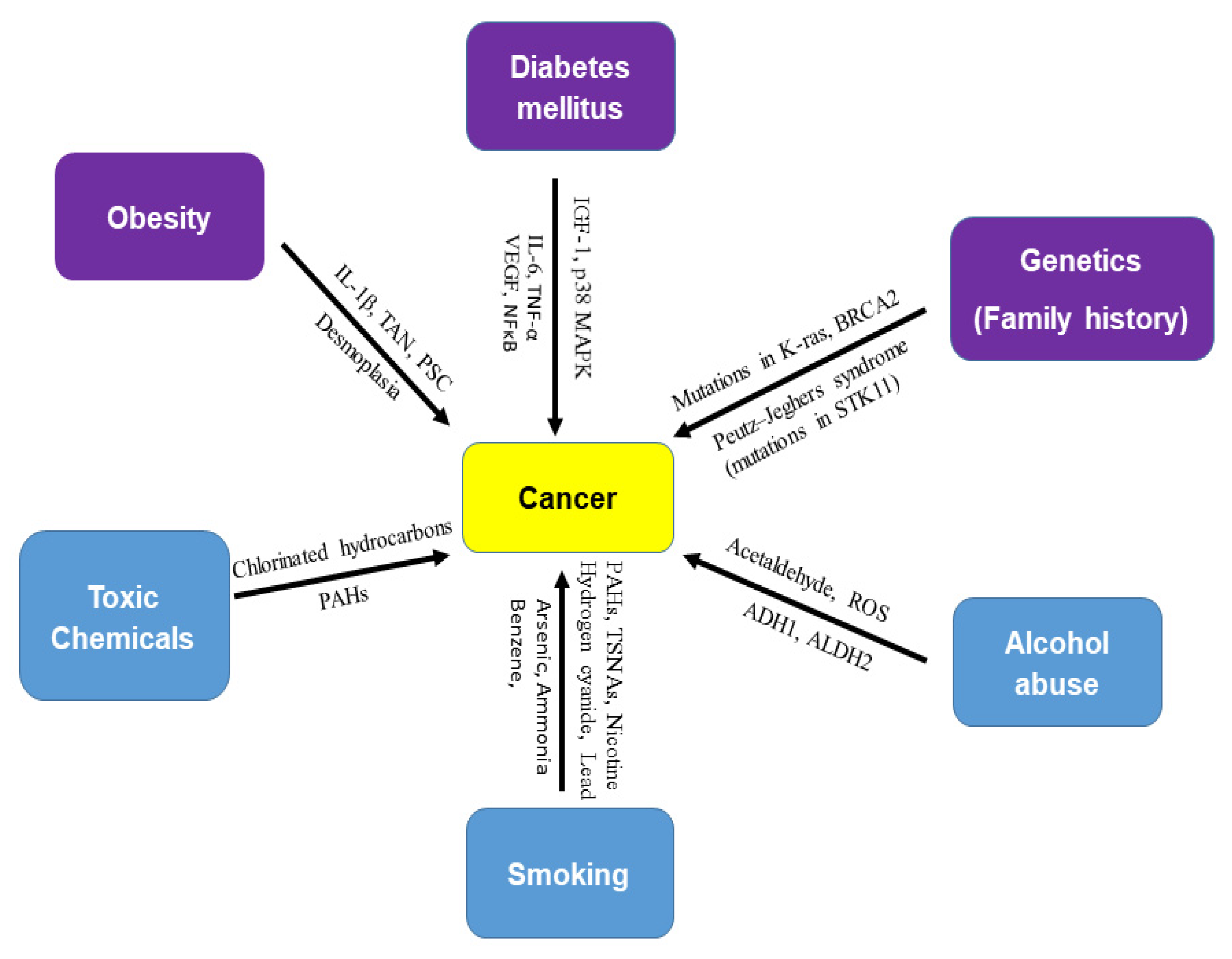
Figure 1. Risk factors of cancer. There are several risk factors for cancer. Obesity (IL-1β, TAN, PSC, desmoplasia), diabetes mellitus (IGF-1, p38 MAPK, IL-6, TNF-β, VEGF, and NF-κB), and genetics (mutations in K-ras, BRCA2, and STK11) are biological risk factors for cancer. Toxic chemicals (chlorinated hydrocarbons and polycyclic aromatic hydrocarbons), alcohol abuse (acetaldehyde, ROS, ADH1, and ALDH2), and smoking (nicotine, hydrogen cyanide, formaldehyde, lead, arsenic, ammonia, benzene, carbon monoxide, nitrosamines, and polycyclic aromatic hydrocarbons), are external or environmental risk factors of cancer. Smoking is known as a strong carcinogen in many cancers. Most cancer cases are attributed to environmental factors but a small percentage are involved in gene mutations and hereditary traces. Peutz–Jeghers syndrome (PJS) is caused by mutations in the tumor suppressor STK11 gene.
2. Diabetes, Alcohol, and Cancer
2.1. Diabetes, Alcohol, and Breast Cancer
2.2. Diabetes, Alcohol, and Pancreatic Cancer
2.3. Diabetes, Alcohol, and Liver Cancer
The major risk factors for hepatocellular carcinoma (HCC), the most frequent histological type of primary liver cancer, are persistent infection with hepatitis B virus (HBV) and hepatitis C virus (HCV), both of which increase the risk of liver cancer to 20-fold [113][42]. Other established risk factors include non-alcoholic fatty liver disease (NAFLD), tobacco smoking, alcohol abuse, exposure to aflatoxin-contaminated food, and some rare inherited disorders, including hereditary hemochromatosis [47,48,114,115,116,117][43][44][45][46][47][48]. Emerging evidence supports a positive association between diabetes and liver cancer [118,119][49][50]. The impact of obesity, genetic factors, and a sedentary lifestyle on liver diseases is shown in Figure 42.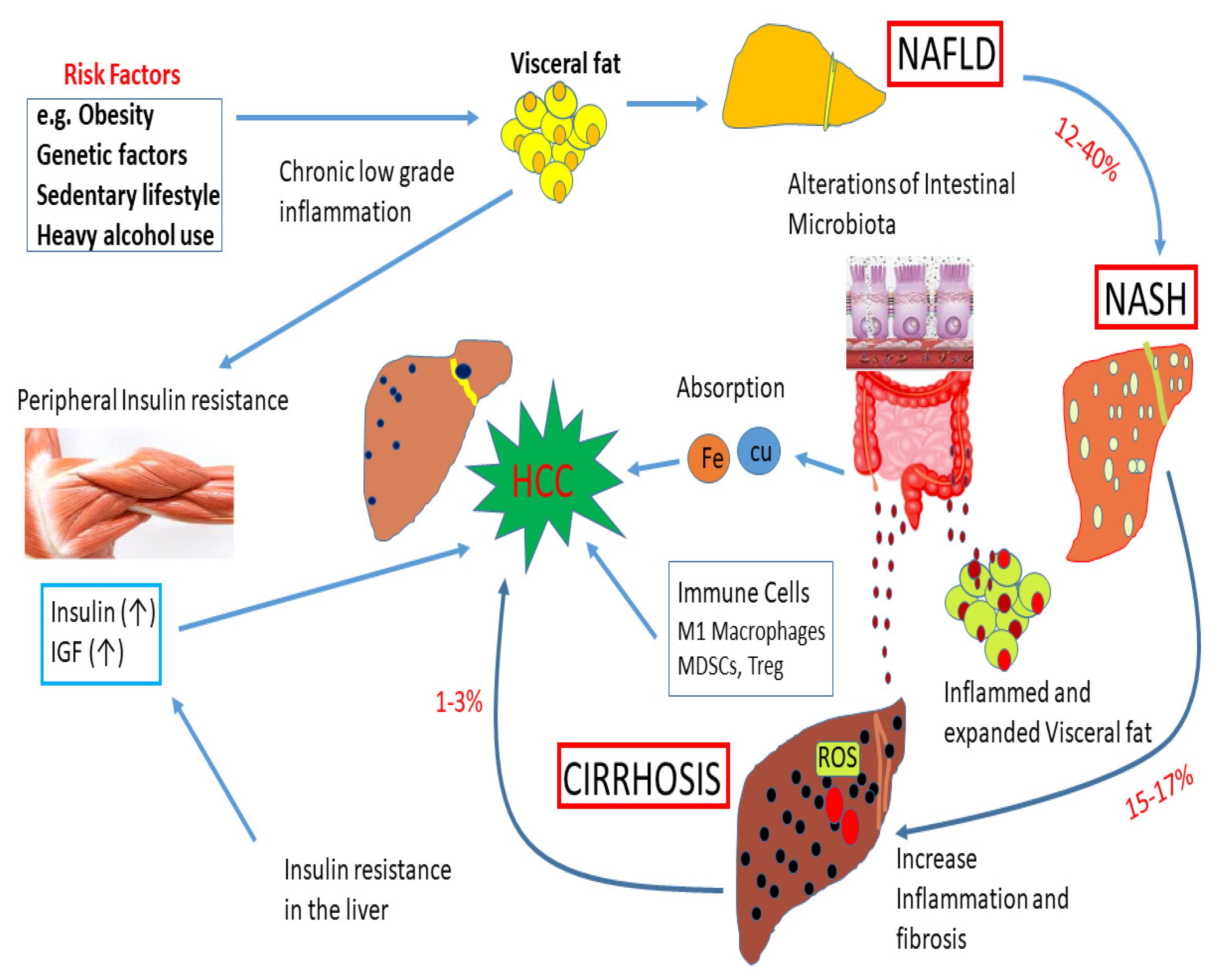 Figure 2. Impact of obesity, genetic factors, and a sedentary lifestyle on liver diseases. Obesity, genetic factors, and a sedentary lifestyle enhance the deposition of visceral fat. These events can play a significant role in the development of non-alcoholic fatty liver disease (NAFLD). About 12–40% of cases of NAFLD develop into NASH. About 15–17% of NASH cases may advance to cirrhosis due to an increase in inflammation and fibrosis. A subset of cirrhosis (1–3%) will finally advance to hepatocellular carcinoma (HCC). High levels of insulin and IGF-1 during insulin resistance can accelerate the development of HCC. Alterations of intestinal microbiota can cause inflammation and visceral fat deposition. The absorption of Fe and Cu in the gut, and immune cells, M1 macrophages, myeloid-derived suppressor cells (MDSCs), and Treg can regulate the development of HCC. In addition, ROS and fatty acid ethyl esters induce cellular damage.
Figure 2. Impact of obesity, genetic factors, and a sedentary lifestyle on liver diseases. Obesity, genetic factors, and a sedentary lifestyle enhance the deposition of visceral fat. These events can play a significant role in the development of non-alcoholic fatty liver disease (NAFLD). About 12–40% of cases of NAFLD develop into NASH. About 15–17% of NASH cases may advance to cirrhosis due to an increase in inflammation and fibrosis. A subset of cirrhosis (1–3%) will finally advance to hepatocellular carcinoma (HCC). High levels of insulin and IGF-1 during insulin resistance can accelerate the development of HCC. Alterations of intestinal microbiota can cause inflammation and visceral fat deposition. The absorption of Fe and Cu in the gut, and immune cells, M1 macrophages, myeloid-derived suppressor cells (MDSCs), and Treg can regulate the development of HCC. In addition, ROS and fatty acid ethyl esters induce cellular damage.
2.4. Diabetes, Alcohol, and Gastric Cancer
The morbidity and mortality rate of gastric cancer has declined rapidly over the past few decades, probably due to the recognition of certain risk factors such as Helicobacter pylori and dietary and environmental risks factors [134][51]. Gastric cancer is more common in men and people aged 50 years or older. Obesity, smoking, and Helicobacter pylori (H. pylori) infection are important risk factors [113,135][42][52]. Few studies have focused on the relationship between DM and the development of gastric cancer. Some systematic meta-analysis reviews have shown the higher risk and mortality of gastric cancer in DM patients [136,137][53][54]. However, others have shown that there is no clear association between DM and the risk of gastric cancer [138,139][55][56]. Recently, it was found that DM increases the risk of early gastric cancer development within an average of 70 months of follow-up [140][57]. All gastric cancers in this study were identified in patients with gastric atrophy, and no cancer was identified in patients without gastric atrophy, which is a typical presentation of H. pylori infection. However, a small percentage of gastric atrophy patients will develop gastric cancer. It was also reported that hyperglycemia and HbA1c increased the risk of gastric cancer induced by H. pylori infection [141][58]. The production of ROS, which causes DNA damage [142][59], is increased in hyperglycemia, and a high glucose level itself has been shown to contribute to DNA damage not only in vitro but also in patients with DM [143][60]. Hyperinsulin in DM has a mitogenic effect by activating the mitogen-activated protein (MAP) or phosphoinositide 3 (PI3) kinase pathway via insulin receptors [144,145][61][62] and the signaling of insulin-like growth factor receptors (IGF-Rs) [146][63].2.5. Diabetes, Alcohol, and Bladder Cancer
Most studies have suggested a positive association between DM and a greater risk of bladder cancer morbidity and mortality, particularly in men [162,163,164][64][65][66]. A recent cumulative meta-analysis showed that DM was positively associated with bladder cancer mortality in both men and women [162][64]. Some studies have provided further evidence of a potential risk of bladder cancer associated with insulin. Insulin enhances bladder cancer cell growth by activating epidermal growth factor and PI3K pathways [165,166][67][68]. Chronic exposure to hyperinsulinemia or hyperglycemia induces tumor cell proliferation and metastasis, which increases insulin-like growth factor (IGF)-1 in diabetic patients, stimulates cellular proliferation, and inhibits apoptosis [167][69]. In DM patients with bladder cancer, the differential regulation of cadherin expression and the degradation of glycosaminoglycans were observed. Furthermore, reduced expression of E-cadherin was associated with poor outcomes in bladder cancer patients, which demonstrated an increase in metastasis. The association between elevated plasma-free fatty acid (FFA) concentrations and insulin resistance has been demonstrated. Although the relationship between FFAs and insulin resistance is complex, a study demonstrated negative correlations between plasma FFA levels and the expression of peroxisome proliferator-activated receptor-gamma cofactor-1 (PGC-1) and nuclear-encoded mitochondrial genes. It was concluded that an increase in FFAs decreases the expression of PGC-1 and nuclear-encoded mitochondrial genes and also enhances the expression of extracellular matrix genes in a manner similar to those of inflammatory diseases [168][70].3. Conclusions
Diabetes, obesity, alcoholism, smoking, chemical exposure, and dietary patterns are closely associated with cancer risk. Although DM and cancer share many common risk factors, several population-based retrospective cohort studies have demonstrated that DM may potentiate gastroenterological carcinogenesis. Thus, avoiding these risks will attenuate the morbidity and mortality of cancers. Furthermore, the discovery of new cancer-related genes is very important for cancer screening strategies and prevention. DM, alcohol misuse, and cancer attract public concern because they are not only medical and health issues but also represent social and financial burdens globally. Therefore, health authorities, social workers, and government agencies should manage DM, alcohol consumption, and cancer in such a way so that these diseases can be prevented and treated simultaneously. Eventually, a better comprehension of pathogenesis and novel therapies should be extensively studied to lower or eliminate DM, alcoholism, and cancer and save human lives.References
- McGinn, O.; Gupta, V.K.; Dauer, P.; Arora, N.; Sharma, N.; Nomura, A.; Dudeja, V.; Saluja, A.; Banerjee, S. Inhibition of hypoxic response decreases stemness and reduces tumorigenic signaling due to impaired assembly of HIF1 transcription complex in pancreatic cancer. Sci. Rep. 2017, 7, 7872.
- Kushwah, V.; Agrawal, A.K.; Dora, C.P.; Mallinson, D.; Lamprou, D.A.; Gupta, R.C.; Jain, S. Novel Gemcitabine Conjugated Albumin Nanoparticles: A Potential Strategy to Enhance Drug Efficacy in Pancreatic Cancer Treatment. Pharm. Res. 2017, 34, 2295–2311.
- Seleznik, G.M.; Reding, T.; Peter, L.; Gupta, A.; Steiner, S.G.; Sonda, S.; Verbeke, C.S.; Dejardin, E.; Khatkov, I.; Segerer, S.; et al. Development of autoimmune pancreatitis is independent of CDKN1A/p21-mediated pancreatic inflammation. Gut 2017, 67, 1663–1673.
- Brooks, P.J.; Zakhari, S. Acetaldehyde and the genome: Beyond nuclear DNA adducts and carcinogenesis. Environ. Mol. Mutagen. 2014, 55, 77–91.
- Srinivasan, S.; Dubey, K.K.; Singhal, R.S. Influence of food commodities on hangover based on alcohol dehydrogenase and aldehyde dehydrogenase activities. Curr. Res. Food Sci. 2019, 1, 8–16.
- Schicchi, A.; Besson, H.; Rasamison, R.; Berleur, M.-P.; Mégarbane, B. Fomepizole to treat disulfiram-ethanol reaction: A case series. Clin. Toxicol. 2019, 58, 922–925.
- Yoshimura, Y.; Nudelman, A.S.; Levery, S.B.; Wandall, H.H.; Bennett, E.P.; Hindsgaul, O.; Clausen, H.; Nishimura, S. Elucidation of the sugar recognition ability of the lectin domain of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase 3 by using unnatural glycopeptide substrates. Glycobiology 2012, 22, 429–438.
- Hao, M.; Wang, W.; Zhao, Y.; Zhang, R.; Wang, H. Pharmacokinetics and tissue distribution of 25-hydroxyprotopanaxadiol, an anti-cancer compound isolated from Panax ginseng, in athymic mice bearing xenografts of human pancreatic tumors. Eur. J. Drug Metab. Pharmacokinet. 2011, 35, 109–113.
- Sheridan, H.; Nestor, C.; O’Driscoll, L.; Hook, I. Isolation, structure elucidation, and cytotoxic evaluation of furanonaphthoquinones from in vitro plantlets and cultures of Streptocarpus dunnii. J. Nat. Prod. 2011, 74, 82–85.
- Abe, R. AGE-RAGE System and Carcinogenesis. Curr. Pharm. Des. 2008, 14, 940–945.
- Colditz, G.A.; Peterson, L.L. Obesity and Cancer: Evidence, Impact, and Future Directions. Clin. Chem. 2018, 64, 154–162.
- Matsumoto, K.; Hasegawa, T.; Ohara, K.; Takei, C.; Kamei, T.; Koyanagi, J.; Takahashi, T.; Akimoto, M. A metabolic pathway for the prodrug nabumetone to the pharmacologically active metabolite, 6-methoxy-2-naphthylacetic acid (6-MNA) by non-cytochrome P450 enzymes. Xenobiotica 2019, 50, 783–792.
- Ye, Y.; Chen, F.; Wu, H.; Lan, S.N.; Jiang, L.R.; Dai, K.K.; Yan, Y.Y.; Yang, L.; Liao, L.C. Relationship between Blood Acetaldehyde Concentration and Psychomotor Function of Individuals with Different ALDH2 Genotypes after Alcohol Consumption. Fa Yi Xue Za Zhi 2019, 35, 576–580.
- Matsumura, Y.; Stiles, K.M.; Reid, J.; Frenk, E.Z.; Cronin, S.; Pagovich, O.E.; Crystal, R.G. Gene Therapy Correction of Aldehyde Dehydrogenase 2 Deficiency. Mol. Ther. Methods Clin. Dev. 2019, 15, 72–82.
- Serio, R.N.; Lu, C.; Gross, S.S.; Gudas, L.J. Different Effects of Knockouts in ALDH 2 and ACSS 2 on Embryonic Stem Cell Differentiation. Alcohol. Clin. Exp. Res. 2019, 43, 1859–1871.
- Lee, Y.J.; Yoo, M.G.; Kim, H.K.; Jang, H.B.; Park, K.J.; Lee, H.J.; Kim, S.G.; Park, S.I. The association between alcohol metabolism and genetic variants of ADH1A, SRPRB, and PGM1 in Korea. Alcohol 2019, 79, 137–145.
- Fu, X.; Yang, H.; Pangestu, F.; Nikolau, B.J. Failure to Maintain Acetate Homeostasis by Acetate-Activating Enzymes Impacts Plant Development. Plant Physiol. 2020, 182, 1256–1271.
- De Hert, M.; Peuskens, J.; Sabbe, T.; Mitchell, A.J.; Stubbs, B.; Neven, P.; Wildiers, H.; Detraux, J. Relationship between prolactin, breast cancer risk, and antipsychotics in patients with schizophrenia: A critical review. Acta Psychiatr. Scand. 2015, 133, 5–22.
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238.
- De Lorgeril, M.; Salen, P. Do statins increase and Mediterranean diet decrease the risk of breast cancer? BMC Med. 2014, 12, 94.
- Maskarinec, G.; Jacobs, S.; Park, S.-Y.; Haiman, C.A.; Setiawan, V.; Wilkens, L.R.; Le Marchand, L. Type II Diabetes, Obesity, and Breast Cancer Risk: The Multiethnic Cohort. Cancer Epidemiol. Biomark. Prev. 2017, 26, 854–861.
- Michels, K.B.; Solomon, C.G.; Hu, F.B.; Rosner, B.A.; Hankinson, S.E.; Colditz, G.A.; Manson, J.E. Type 2 Diabetes and Subsequent Incidence of Breast Cancer in the Nurses’ Health Study. Diabetes Care 2003, 26, 1752–1758.
- Woźniak, M.K.; Wiergowski, M.; Namieśnik, J.; Biziuk, M. Biomarkers of Alcohol Consumption in Body Fluids—Possibilities and Limitations of Application in Toxicological Analysis. Curr. Med. Chem. 2019, 26, 177–196.
- Chen, J.; Qin, C.; Zhou, Y.; Chen, Y.; Mao, M.; Yang, J. Metformin may induce ferroptosis by inhibiting autophagy via lncRNA H19 in breast cancer. FEBS Open Bio. 2021.
- Shi, B.; Hu, X.; He, H.; Fang, W. Metformin suppresses breast cancer growth via inhibition of cyclooxygenase-2. Oncol. Lett. 2021, 22, 1–14.
- Yenmiş, G.; Beşli, N.; Saraç, E.Y.; Emre, F.S.H.; Şenol, K.; Sultuybek, G.K. Metformin promotes apoptosis in primary breast cancer cells by downregulation of cyclin D1 and upregulation of P53 through an AMPK-alpha independent mechanism. Turk. J. Med. Sci. 2021, 51, 826–834.
- Dam, M.K.; Hvidtfeldt, U.A.; Tjonneland, A.; Overvad, K.; Grønbæk, M.K.; Tolstrup, J. Five year change in alcohol intake and risk of breast cancer and coronary heart disease among postmenopausal women: Prospective cohort study. BMJ 2016, 353, i2314.
- Leung, T.; Rajendran, R.; Singh, S.; Garva, R.; Krstic-Demonacos, M.; Demonacos, C. Cytochrome P450 2E1 (CYP2E1) regulates the response to oxidative stress and migration of breast cancer cells. Breast Cancer Res. 2013, 15, R107.
- Larsen, S.B.; Kroman, N.; Ibfelt, E.H.; Christensen, J.; Tjønneland, A.; Dalton, S.O. Influence of metabolic indicators, smoking, alcohol and socioeconomic position on mortality after breast cancer. Acta Oncol. 2015, 54, 780–788.
- Steele, V.E.; Lubet, R.A. The Use of Animal Models for Cancer Chemoprevention Drug Development. Semin. Oncol. 2010, 37, 327–338.
- Heretsch, P.; Tzagkaroulaki, L.; Giannis, A. Modulators of the hedgehog signaling pathway. Bioorg. Med. Chem. 2010, 18, 6613–6624.
- Kumar, D.; Singh, G.; Sharma, P.; Qayum, A.; Mahajan, G.; Mintoo, M.; Singh, S.K.; Mondhe, D.M.; Bedi, P.; Jain, S.K.; et al. 4-aryl/heteroaryl-4H-fused Pyrans as Anti-proliferative Agents: Design, Synthesis and Biological Evaluation. Anti-Cancer Agents Med. Chem. 2018, 18, 57–73.
- Poudel, B.K.; Gupta, B.; Ramasamy, T.; Thapa, R.K.; Pathak, S.; Oh, K.T.; Jeong, J.-H.; Choi, H.-G.; Yong, C.S.; Kim, J.O. PEGylated thermosensitive lipid-coated hollow gold nanoshells for effective combinational chemo-photothermal therapy of pancreatic cancer. Colloids Surfaces B Biointerfaces 2017, 160, 73–83.
- Goodenberger, M.H.; Wagner-Bartak, N.A.; Gupta, S.; Liu, X.; Yap, R.Q.; Sun, J.; Tamm, E.P.; Jensen, C.T. Computed Tomography Image Quality Evaluation of a New Iterative Reconstruction Algorithm in the Abdomen (Adaptive Statistical Iterative Reconstruction–V) a Comparison With Model-Based Iterative Reconstruction, Adaptive Statistical Iterative Reconstruction, and Filtered Back Projection Reconstructions. J. Comput. Assist. Tomogr. 2018, 42, 184–190.
- Gupta, R.; Amanam, I.; Chung, V. Current and future therapies for advanced pancreatic cancer. J. Surg. Oncol. 2017, 116, 25–34.
- Vashi, P.G.; Virginkar, N.; Popiel, B.; Edwin, P.; Gupta, D. Incidence of and factors associated with catheter-related bloodstream infection in patients with advanced solid tumors on home parenteral nutrition managed using a standardized catheter care protocol. BMC Infect. Dis. 2017, 17, 1–9.
- Gupta, N.; Rath, S.K.; Singh, J.; Qayum, A.; Singh, S.; Sangwan, P.L. Synthesis of novel benzylidene analogues of betulinic acid as potent cytotoxic agents. Eur. J. Med. Chem. 2017, 135, 517–530.
- Dauer, P.; Gupta, V.K.; McGinn, O.; Nomura, A.; Sharma, N.; Arora, N.; Giri, B.; Dudeja, V.; Saluja, A.K.; Banerjee, S. Inhibition of Sp1 prevents ER homeostasis and causes cell death by lysosomal membrane permeabilization in pancreatic cancer. Sci. Rep. 2017, 7, 1–9.
- Dangroo, N.A.; Singh, J.; Rath, S.K.; Gupta, N.; Qayum, A.; Singh, S.; Sangwan, P.L. A convergent synthesis of novel alkyne–azide cycloaddition congeners of betulinic acid as potent cytotoxic agent. Steroids 2017, 123, 1–12.
- Fomenko, E.V.; Chi, Y. Mangiferin modulation of metabolism and metabolic syndrome. BioFactors 2016, 42, 492–503.
- Stratton-Powell, A.A.; Pasko, K.M.; Brockett, C.L.; Tipper, J.L. The Biologic Response to Polyetheretherketone (PEEK) Wear Particles in Total Joint Replacement: A Systematic Review. Clin. Orthop. Relat. Res. 2016, 474, 2394–2404.
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044.
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332–349.
- Wang, W.; Wang, C.; Xu, H.; Gao, Y. Aldehyde Dehydrogenase, Liver Disease and Cancer. Int. J. Biol. Sci. 2020, 16, 921–934.
- Vatamaniuk, M.Z.; Gupta, R.K.; Lantz, K.A.; Doliba, N.M.; Matschinsky, F.M.; Kaestner, K.H. Foxa1-Deficient Mice Exhibit Impaired Insulin Secretion due to Uncoupled Oxidative Phosphorylation. Diabetes 2006, 55, 2730–2736.
- Chuang, S.-C.; Lee, Y.-C.A.; Wu, G.-J.; Straif, K.; Hashibe, M. Alcohol consumption and liver cancer risk: A meta-analysis. Cancer Causes Control. 2015, 26, 1205–1231.
- Taniai, M. Alcohol and hepatocarcinogenesis. Clin. Mol. Hepatol. 2020, 26, 736–741.
- Thylur, R.P.; Roy, S.K.; Shrivastava, A.; LaVeist, T.A.; Shankar, S.; Srivastava, R.K. Assessment of risk factors, and racial and ethnic differences in hepatocellular carcinoma. JGH Open 2020, 4, 351–359.
- Makol, A.; Kanthaje, S.; Dhiman, R.K.; Kalra, N.; Chawla, Y.K.; Chakraborti, A. Association of liver cirrhosis severity with type 2 diabetes mellitus in hepatocellular carcinoma. Exp. Biol. Med. 2017, 243, 323–326.
- Lim, H.-W.; Bernstein, D.E. Risk Factors for the Development of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Including Genetics. Clin. Liver Dis. 2018, 22, 39–57.
- Siegel, A.B.; Goyal, A.; Salomao, M.; Wang, S.; Lee, V.; Hsu, C.; Rodriguez, R.; Hershman, D.L.; Brown, R.S., Jr.; Neugut, A.I.; et al. Serum Adiponectin Is Associated with Worsened Overall Survival in a Prospective Cohort of Hepatocellular Carcinoma Patients. Oncology 2014, 88, 57–68.
- Correa, P.; Piazuelo, M.B. Helicobacter pylori Infection and Gastric Adenocarcinoma. US Gastroenterol. Hepatol. Rev. 2011, 7, 59–64.
- Tian, T.; Zhang, L.Q.; Ma, X.H.; Zhou, J.N.; Shen, J. Diabetes Mellitus and Incidence and Mortality of Gastric Cancer: A Meta-Analysis. Exp. Clin. Endocrinol. Diabetes 2012, 120, 217–223.
- Yoon, J.M.; Son, K.Y.; Eom, C.S.; Durrance, D.; Park, S.M. Pre-existing diabetes mellitus increases the risk of gastric cancer: A meta-analysis. World J. Gastroenterol. 2013, 19, 936–945.
- Ge, Z.; Ben, Q.; Qian, J.; Wang, Y.; Li, Y. Diabetes mellitus and risk of gastric cancer. Eur. J. Gastroenterol. Hepatol. 2011, 23, 1127–1135.
- Jayaprakash, V.; Marimuthu, S.P.; Vijayaragavan, P.; Moysich, K.B. Diabetes mellitus and gastric carcinoma: Is there an association? J. Carcinog. 2011, 10, 30.
- Sekikawa, A.; Fukui, H.; Maruo, T.; Tsumura, T.; Okabe, Y.; Osaki, Y. Diabetes mellitus increases the risk of early gastric cancer development. Eur. J. Cancer 2014, 50, 2065–2071.
- Ikeda, K.; Kobayashi, M.; Someya, T.; Saitoh, S.; Hosaka, T.; Akuta, N.; Suzuki, F.; Suzuki, Y.; Arase, Y.; Kumada, H. Occult hepatitis B virus infection increases hepatocellular carcinogenesis by eight times in patients with non-B, non-C liver cirrhosis: A cohort study. J. Viral Hepat. 2009, 16, 437–443.
- Kountouras, J.; Boura, P.; Lygidakis, N.J. Omeprazole and regulation of cytokine profile in Helicobacter pylori-infected patients with duodenal ulcer disease. Hepatogastroenterology 2000, 47, 1301–1304.
- Dandona, P.; Thusu, K.; Cook, S.; Snyder, B.; Makowski, J.; Armstrong, D.; Nicotera, T. Oxidative damage to DNA in diabetes mellitus. Lancet 1996, 347, 444–445.
- Vigneri, R. Diabetes therapy and cancer risk. Nat. Rev. Endocrinol. 2009, 5, 651–652.
- Novosyadlyy, R.; Leroith, D. Hyperinsulinemia and type 2 diabetes: Impact on cancer. Cell Cycle 2010, 9, 1449–1450.
- Pollak, M. Targeting insulin and insulin-like growth factor signalling in oncology. Curr. Opin. Pharmacol. 2008, 8, 384–392.
- Zhu, Z.; Zhang, X.; Shen, Z.; Zhong, S.; Wang, X.; Lu, Y.; Xu, C. Diabetes Mellitus and Risk of Bladder Cancer: A Meta-Analysis of Cohort Studies. PLoS ONE 2013, 8, e56662.
- Newton, C.C.; Gapstur, S.M.; Campbell, P.T.; Jacobs, E.J. Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int. J. Cancer 2013, 132, 2186–2191.
- Xu, Y.; Huo, R.; Chen, X.; Yu, X. Diabetes mellitus and the risk of bladder cancer. Medicine 2017, 96, e8588.
- Ornskov, D.; Nexo, E.; Sorensen, B.S. Insulin induces a transcriptional activation of epiregulin, HB-EGF and amphiregulin, by a PI3K-dependent mechanism: Identification of a specific insulin-responsive promoter element. Biochem. Biophys. Res. Commun. 2007, 354, 885–891.
- Ornskov, D.; Nexo, E.; Sorensen, B.S. Insulin-induced proliferation of bladder cancer cells is mediated through activation of the epidermal growth factor system. FEBS J. 2006, 273, 5479–5489.
- Richardson, D.W.; Vinik, A.I. Metabolic Implications of Obesity: Before and After Gastric Bypass. Gastroenterol. Clin. N. Am. 2005, 34, 9–24.
- Richardson, D.K.; Kashyap, S.; Bajaj, M.; Cusi, K.; Mandarino, S.J.; Finlayson, J.; DeFronzo, R.A.; Jenkinson, C.P.; Mandarino, L.J. Lipid Infusion Decreases the Expression of Nuclear Encoded Mitochondrial Genes and Increases the Expression of Extracellular Matrix Genes in Human Skeletal Muscle. J. Biol. Chem. 2005, 280, 10290–10297.
