The aim of this work is to investigate the preparation, the optical properties and the stability over time of a colloidal oraganic-inorganic hybrid perovskite (CH3NH3PbBr3)/random copolymer P(MMA-co-DMAEMA) system. Different ratios of perovskite to copolymer were used to study its effect on stability and properties. The optical properties were investigated by UV-Vis and Fluorescence Spectroscopy. Dynamic light scattering was used to determine the size, and the polydispersity of the colloidal hybrid particles; while morphology was investigated by transmission electron microscopy. Photoluminescence decay studies revealed the interaction of the random copolymer with the perovskite. Finally, thin-films were prepared to investigate the optical properties of thin hybrid films was observed under certain conditions.
1. Introduction
Organic–inorganic hybrid perovskites (Hyb-Per) are an emerging class of solution processable semiconducting materials that combine the favorable properties of the inorganic semiconductor with the flexibility and low-temperature process ability of the organic material
[1,2][1][2]. They show excellent optoelectronic properties, such as a high and balanced carrier mobility
[3[3][4],
4], long carrier diffusion length
[5], tunable bandgap
[6], high photoluminescence quantum yield, and large light absorption coefficient in the UV–Vis range
[7,8][7][8]. By choosing the appropriate amine-metal-halogen combination, the optical band gap, as well as their emission and absorption spectra, can be controlled throughout the entire visible range
[9,10,11][9][10][11]. The Hyb-Per can be prepared with quick and easy synthetic procedures and their thin-films can be produced easily even on an industrial scale, using low-cost film-deposition techniques that allow the adjustment in morphology, composition, and crystalline properties. Because of their easy and quick synthesis procedures and their optical and semiconducting properties, Hyb-Per have already shown a tremendous potential for use in optoelectronic devices
[12] such as light-emitting diodes (LEDs)
[13], photodetectors
[14], lasers
[15], and field-effect transistors (FETs)
[16]. Especially, their use for photovoltaic applications took the photovoltaic community by storm with an improvement of the solar to electric conversion efficiency from 3.8 to 22.1% in just six years
[17].
Lately, the expanded use of LEDs for artificial lighting and imaging, due to better quality and significant energy savings, increase the effort for the preparation of new materials to replace the expensive inorganic semiconductors prepared with vacuum-based epitaxial growth on expensive rigid substrates. The high photoluminescence quantum yield, narrow full width to half-maximum (≈20 nm)
[18], and the easiness of Hyb-Per synthesis and their film preparation has placed them among the strong candidates as materials for light-emitting diode (PLED) devices and display applications
[13].
The crystal structure of (CH3NH3)PbX3, (X: Cl, Br, I) consists of two different components, an inorganic network of corner sharing PbX6 octahedra and organic cations (CH3NH
3+) in the voids of the network. By mixing and grinding the precursor salts at room temperature, nanocrystals of the organic–inorganic hybrid perovskites are formed, characteristic of the easiness with which the organic cations can diffuse into the inorganic framework. However, the size and quality of the nanocrystals prepared by this method is suffering from repeatability, due to the lack of precise control of experimental conditions. The most common synthetic route for the formation of hybrid perovskite nanocrystals is the ligand-assisted reprecipitation method. Factors such as the size and the dimensionality of nanocrystals can be adjusted by changing the synthetic procedure resulting in different optical features
[19,20][19][20]. The possibility of stable hybrid perovskite nanocrystals dispersed in solution or in a polymer matrix would enable the preparation of new optoelectronic devices
[21]. The preparation of Hyb-Per in the form of colloidal nanocrystals solutions is of great scientific and technological interest
[22]. One of the main issues that remain to be solved is their instability towards air, temperature, light irradiation, and water. According to the ligand-assisted reprecipitation method, the inorganic metal and ammonium, halide salts were dissolved in a polar solvent and injected into a nonpolar solvent, resulting in an instantaneous formation of nanocrystals. The presence of a good capping agent is necessary to stabilize the newly formed nanocrystals. Lately, few attempts have been made to stabilize them, using amines
[23], oleic acid
[24], or polymers
[25] as protective agents. These protected colloidal nanocrystals present increased stability over time enhanced exciton stability and many times greater photoluminescence than the bulk material
[25], also the wavelength of the excitonic absorption and photoluminescence can be tailored by controlling the size of the nanoparticles
[9,26][9][26].
Amphiphilic copolymers have been used extensively due to their self-assembling behavior into nanoparticles when inserted in a selective solvent
[27,28][27][28]. Different architectures such as random, diblock, multiblock, star, and graft copolymers can be made by regulating the synthesis procedures
[29]. The copolymer architecture and the suitability of the solvent determine the nanostructure morphology. For example, block copolymers form well-controlled and organized nanoparticles, while random copolymers form more unusual and irregular morphologies
[30,31,32][30][31][32]. Hybrid, well-defined nanostructures can be accomplished by combining/mixing self-assembling of amphiphilic copolymers with inorganic, semiconducting materials. Therefore, the formed hybrid materials display improved mechanical, optical, and absorptive properties and greater stability over time
[19,33,34][19][33][34].
2. Hybrid Nanoparticle Preparation and Dynamic Light Scattering Measurements
P(MMA-co-DMAEMA) random copolymer was chosen as a colloidal stabilizer since the MMA segments form glassy nonpolar/hydrophobic nanodomains while the DMAEMA segments form polar/hydrophilic nanodomains in aqueous or organic solvent solutions of respective polarity. Both segments can interact with entities of similar polarity and tertiary amine groups on DMAEMA segments can also exert complexation functions towards perovskite entities.
When an amphiphilic block or random copolymer is inserted in a nonpolar medium, the hydrophilic part forms the cores of the obtained self-assembled nanoparticles while the hydrophobic part surrounds the cores. The reverse phenomenon is observed when the copolymer is inserted in a polar solvent. Thus, the copolymer matrix functions as a protective agent in order to avoid the precipitation of the crystalline CH
3NH
3PbBr
3 and keeps the hybrid perovskite/copolymer stable in solution. Formation of the core allows the encapsulation of the CH
3NH
3PbBr
3 nanocrystals, while MMA units cover the core in order to protect from precipitation and stabilize the CH
3NH
3PbBr
3 nanocrystals and the hybrid ensemble in nonpolar solvents. Specifically, P(MMA-co-DMAEMA) copolymers when inserted in an organic nonpolar medium such as toluene, they self-organize into nanoparticles where the DMAEMA segment forms the core and the MMA segment encompasses the polar DMAEMA segments. When the CH
3NH
3PbBr
3 nanocrystals are encapsulated into the DMAEMA core, intermolecular interactions develop between the DMAEMA part and the perovskite nanocrystal, while the MMA part offers colloidal stability to the system.
In order to gain information about the size, the size polydispersity, and the stability of perovskite/copolymer nanoparticles, DLS measurements were performed. The results are presented in
Table 1 and
Table 2.
Table 1. Dynamic light scattering results for solution 1a, 1b, 1c, 1d, on the day they were prepared.
| Sample |
C | perov in DMF | (mM) |
C | copol in toluene | (g/mL) |
R | h | | a | [35] (nm) |
| 1a |
6.25 |
3.125 × 10 | −3 |
Table 2. Intensity and Rh values of samples 1a, 1b, 1c, 1d on 1st day, and after 7, 20, 30 days.
| |
Sample 1a |
Sample 1b |
Sample 1c |
Sample 1d |
| 2.4 |
| Day |
Intensity | a | (kc/s) |
Rh | a | [36] (nm) |
Intensity | a | (kc/s) |
Rh | a | [36] (nm) |
Intensity | a | (kc/s) |
Rh | a | [36] (nm) |
Intensity | a | (kc/s) |
Rh | a | [36] (nm) |
| 1b |
| 1st | 12.5 |
283.125 × 10 | −3 |
58 |
| 2.45 |
124 |
58 |
2994 |
45.9 |
18,387 |
64 |
1c |
25.0 |
3.125 × 10 | −3 |
46 |
| 7th |
37 |
7.5 |
99 |
51 |
880 |
46 |
14,387 |
64 |
1d |
50.0 |
3.125 × 10 | −3 |
64 |
| 20th |
34 |
- |
53 |
38 |
612 |
47.2 |
3277 |
60 |
2a |
6.25 |
- |
N/A |
| 30th |
- |
- |
51 |
53 |
385 |
46.5 |
2590 |
2b |
12.5 |
- |
150 |
| 57 |
2c |
25.0 |
- |
123 |
| 2d |
50.0 |
- |
117 |
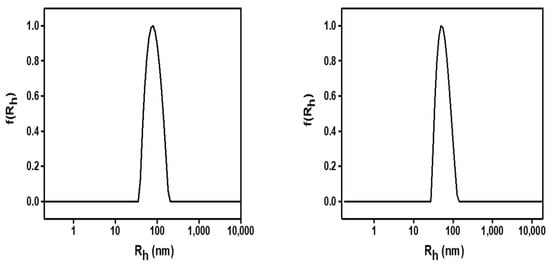
In all cases, a single peak from CONTIN
[36], analysis of the DLS correlation functions is observed (
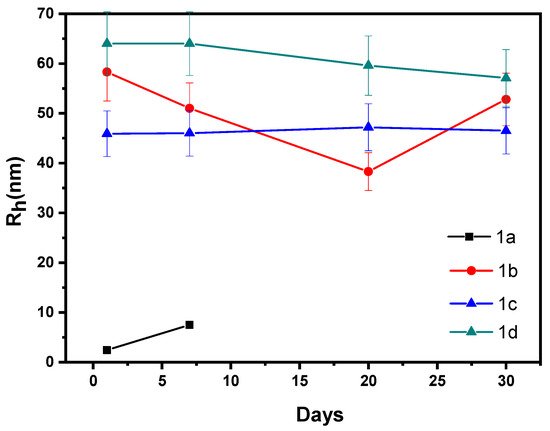
Figure 1), indicating the homogeneity of the system in each case. Moreover, by increasing the concentration of the perovskite solution, no precipitation has occurred, suggesting that colloidal stable nanoparticles can be formed in all cases. It is observed that the nanoparticles formed, without the presence of polymer, present increased hydrodynamic radius values, relative to the corresponding ones, to which the copolymer has been added. The measurements repeated once a week for a period of one month (
Figure 2). In the case of series 1, a single peak from the CONTIN analysis was observed at all times and no precipitation or significant change in the radius of the nanoparticles occurred, except for the solution 1a, which contains the lower amount of perovskite, displaying once more the stability of the system over time. The low R
h value (2.4 nm) at angle of 90 degrees as well as the low scattering intensity value (28 kc/s) of Sample 1a are attributed to the fact that the concentration value of the perovskite solution in this case is not adequate in order for mixed perovskite/random copolymer nanoparticles to be formed. Therefore, both the R
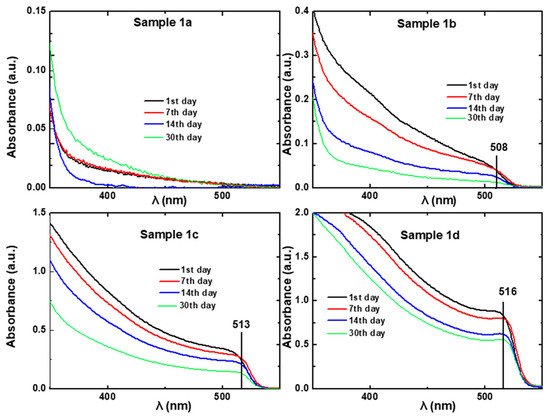
h and the scattering intensity values are due to the single chain formation of the random copolymer in toluene. According to UV-Vis spectra (
Figure 3), the sample does not exhibit excitonic absorption and in combination with the low scattering light intensity, it is possible that the perovskite nanocrystals were retained on the filter. On the seventh day, an increase in the scattering light intensity value, which is accompanied with an increase in the Rh value, demonstrates the formation of nanoaggregates. Therefore, it can be assumed that the self-assembly of the random copolymer and perovskite into nanoparticles is a slow process that takes about a week long in order to be completed. On the 20th day, the intensity value has been decreased due to the precipitation of the nanoparticles. The size distribution analysis is not possible due to the presence of dust. It is possible to suppose that a certain concentration and ratio of the components is needed in order to form stable hybrid nanoparticles under the conditions utilized in this study.
Figure 1. Size distribution from CONTIN analysis for Sample 1b (left) and 1d (right) at 90°. Data was collected on day 1.
Figure 2. Rh values fluctuation for Samples 1a, 1b, 1c, and 1d from day 1 to day 30, as it was obtained from dynamic light scattering measurements.
Figure 3. UV-Vis absorption spectra of solutions (1a), (1b), (1c), and (1d) from 1st (black line), 7th (red line) 14th (Blue line), and 30th (green line) day.
Concerning solution 1b, after the filtration, the solution was discolored and the larger perovskite particles were retained on the filter. We assume that certain perovskite particles were not encapsulated in the polymeric nanoparticles and this is the reason why they were retained on the filter. On the first day, the intensity and R
h values shows the presence of polydispersed nanoparticles in toluene. The perovskite nanocrystals were encapsulated in the polymeric nanoaggregates. At longer times, the intensity values decrease due possibly to particle precipitation.
No change of color was observed for solution 1c after filtration. On the first day, the intensity value was 2994 kcps (kilophotons per second) and the Rh was 46 nm. Based on the intensity value and the excitonic absorption, which is displayed in the UV-vis spectrum (
Figure 3 it can be assumed that the encapsulation of the perovskite nanocrystals into the polymeric nanoparticles was successful. The 1c polymer/perovskite hybrid nanosystem exhibits high size homogeneity. At latter times, the intensity value decreases due to at least partial precipitation of the mixed polymer/perovskite nanostructures, while the hydrodynamic radius appears to be relatively stable. The high intensity value on day 7th, 20th, and 30th is due to the sufficient number of nanoparticles that are still dispersed in the solution, even though precipitation of certain nanoparticles must have occurred. Sample 1c seems to exhibit greater colloidal stability than Samples 1a and 1b, over time.
No change of color was observed also in the case of Sample 1d. This sample presents the highest size homogeneity of all. Moreover, Sample 1d exhibits the greatest intensity value of all, as high-mass mixed nanostructures are presumably formed due to the higher amount and ratio of the perovskite component in the mixed solutions. The encapsulation of perovskite nanocrystals into the polymeric nanoparticles appears to be successful. The sample appears to be stable over time, as long as the hydrodynamic radius is observed, but the scattering intensity value is partially reduced. The latter may be a sign of partial precipitation. Although a number of polymer/perovskite mixed nanoparticles precipitated, the intensity value seems to be high (but decreased compared with day 1) as there are still a large number of dispersed hybrid nanoparticles in toluene.
To sum up, for Samples 1c and 1d, formation of stable hybrid polymer/perovskite nanoparticles was observed with significant colloidal stability. The scattering light intensity is high in both cases, while Sample 1d exhibits the highest value. Both samples present great stability over time, as hydrodynamic radius measurements indicate. Specifically, Sample 1c appears to be more stable than 1d in terms of both size and intensity value. Both samples display high size homogeneity, but Sample 1d has lower polydispersity index, indicating that Sample 1d is more homogeneous than 1c. Finally, the preparation procedure was repeated several times. The results from DLS experiments were similar in all the cases, indicating the repeatability of the synthesis protocol.
For the samples prepared in the absence of copolymer (Samples 2a, 2b, 2c, 2d), formation of large nanoparticles (R
h > 100 nm) was observed, which were not stable and precipitated after some hours.
3. Optical Properties
3.1. Perovskite/Polymer Solutions
According to UV-Vis spectra (
Figure 3), the sample with the lowest concentration (1a) does not exhibit excitonic absorption even from the first day. The other three samples (1b, 1c, 1d) exhibit excitonic absorption with intensities that increase as the concentration of the perovskite is increased. Moreover, a redshift is observed when the concentration of the perovskite solution is increased. Specifically, the peak of exitonic absorption of Sample 1b is observed at λ
max ~ 508 nm, of Sample 1c at λ
max ~ 513 nm and of Sample 1d at λ
max ~ 516 nm, indicating the formation of larger nanocrystals as the perovskite concentration increases. The exitonic peaks are slightly red shifted with the time, pointing out that the size of the nanocrystals is increased as time passes, possibly due to further reorganization phenomena. Those peaks are blue shifted compared to those of film and single crystal CH
3NH
3PbBr
3 [37] (520
[38] and 550 nm
[39] respectively) mainly due to the particle-size quantum confinement effect.
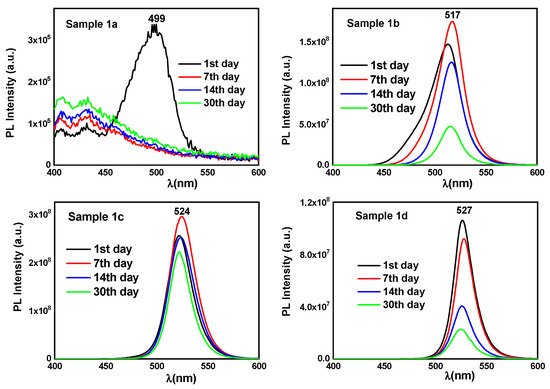
The sample with the lowest concentration (1a) shows a weak excitonic emission peak at 499 nm that is disappeared after some days (
Figure 4). All the other samples (1b, 1c, 1d) exhibit strong excitonic emission peaks. As in the case of absorbance spectra, all the emission peaks are red shifted compared to CH
3NH
3PbBr
3 film and single crystal. Also, a redshift of the emission peaks is observed as the perovskite concentration gets higher, possibly due to the formation of nanocrystals of larger size. Moreover,
Figure 4 suggests that photoluminescence originated from CH
3NH
3PbBr
3 immediately after the synthesis, which indicates that the perovskite nanocrystals have been formed. The photoluminescence intensity of Samples 1b, 1c, and 1d remain strong for the whole 30 days period of study, although there is a decrease of the intensity. The small Stokes shift of the samples indicate that the PL emission originates from direct exciton recombination. Similar spectra were obtained by CH
3NH
3PbBr
3 nanocrystals prepared by different methods.
Figure 4. Fluorescence emission spectra of solutions (1a), (1b), (1c), and (1d) from 1st (black line), 7th (red line), 14th (blue line), and 30th (green line) day.
To the analogues blank solutions without the polymer addition, precipitation observed after some hours. Consequently, no absorption peaks and very weak fluorescence peaks were observed. Decrease of the absorption and emission values, as time passes, comprise an indication that the colloidal stability of perovskite/polymer hybrid system is decreased. The decrease of the colloidal stability is also confirmed by the dynamic light scattering results, where the value of the hydrodynamic radius is also increased. On the other hand, for the samples of series 2, precipitation occurs after some hours and do not exhibit excitonic absorption or PL emission, indicating that no stable nanocrystals were rated in the absence of copolymer.
3.2. Time-Resolved Photoluminescence Decay Studies
In order to study further the photoluminescence properties of the perovskite/polymer hybrid solutions, photoluminescence decay studies were performed and compared with those of the perovskite solution without the presence of the random copolymer dispersed in toluene (blank experiment).
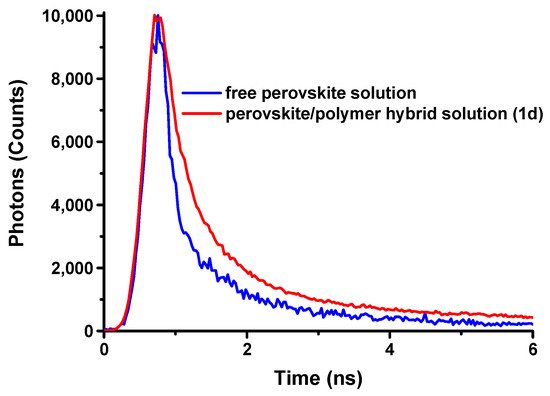
Firstly, fresh solutions of Samples 1d and 2d were prepared and the fluorescence lifetime profiles for both perovskite/polymer hybrid system and free perovskite dispersed in toluene were obtained immediately and analyzed (
Figure 5). The analysis of the time profile of the fluorescence decay at 375 nm for the free perovskite dispersed in toluene showed only one component with 1.14 ns lifetime, while two components were detected for the perovskite/polymer hybrid material; a faster one with 90 ps lifetime (18%), assigned to perovskite nanocrystals, which probably were not encapsulated into the polymer containing nanoparticles, and a slower one in a much higher percentage (82%) with 9.8 ns lifetime, attributed to the hybrid nanomaterial. These values are significantly lower than those of bulk films (~100 ns)
[40,41][40][41]. A possible scenario is that the short (90 ps) lifetime corresponds to a low ratio (18%) of perovskite nanocrystals, which probably were not encapsulated into the polymer containing nanoparticles, and thus they are not colloidal stable and exhibit short lifetime properties. The longer lifetime in a much higher percentage (82%) with 9.8 ns lifetime is assigned to the hybrid nanomaterial possibly due to a reduction of the defect density that the incorporation of the polymer matrix established. The polymer matrix probably decreases the defect sites and removes trapped states, a fact that leads to the elongation of photoluminescence decay time
[42].
Figure 5. Lifetime decays of perovskite solution of C = 50.08 mM dispersed in toluene, without the presence of the random copolymer, (blue line) and Sample 1d (red line).
As a result, photoluminescence decay assays reinforce the belief that the PMMA-co- PDMAEMA random copolymer functions as a protective matrix against perovskite precipitation and also provides colloidal and temporal stability.
3.3. Perovskite/Polymer Hybrid Thin-Films
Furthermore, we investigate the photoluminescence intensity of perovskite/polymer thin films (Samples TFa, TFb, TFc, TFd), which were prepared by homogenous coating of Samples 1a, 1b, 1c, 1d on a glass surface, respectively. The aim of this experiment is to investigate if the copolymer/perovskite hybrid system is capable of exhibiting emissions in absence of toluene and therefore could be applied as active components of an OLED device. UV-Vis absorption and fluorescence emission were studied at regular time intervals in order to determine time stability of the thin-films optical/photophysical properties. The results are presented in
Table 3.
Table 3. Thin-Film samples, their precursor solutions, thickness of the films, and concentration of perovskite in each sample.
Precursor
Solution |
Thin-Film
Sample Code |
C Perovskite
in DMF (mM) |
Thickness (μm) |
| 1a |
TFa |
6.26 |
1500.3 |
| 1b |
TFb |
12.52 |
4900.4 |
| 1c |
TFc |
25.04 |
1700.8 |
| 1d |
TFd |
50.08 |
3400.2 |
A redshift is observed in both absorbance and emission spectra when the concentration of the perovskite in the film is increased due to the increase of the nanocrystals size (
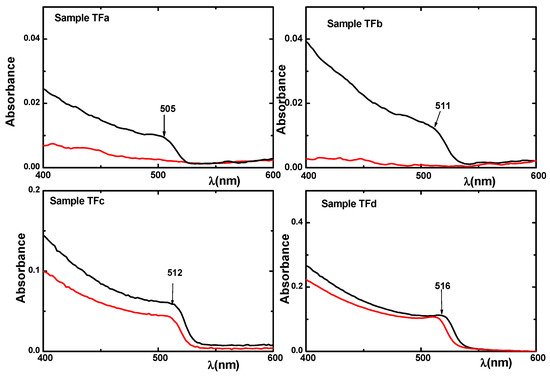
Figure 6 and
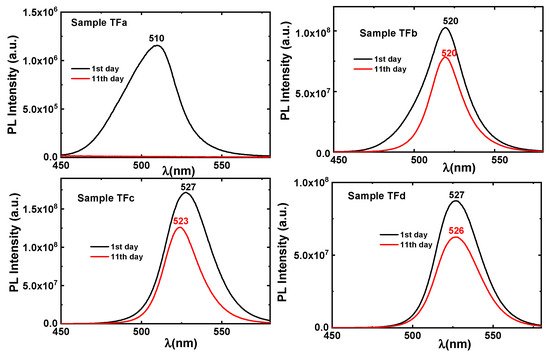
Figure 7). In the case of sample TFa, the UV-Vis absorption spectrum exhibits an excitonic peak at λ = 505 nm, and an emission peak at 510 nm, both peaks disappeared at the second measurement (11th day). The same behavior was observed for the TFb sample, but although the excitonic absorption at ~516 nm has disappeared, on the 11th day there was still a significant emission signal (
Figure 6 and
Figure 7). The characteristic peaks due to exciton appear at the fresh films of the hybrid samples TFa, TFb, TFc, and TFd (
Figure 6 and
Figure 8).
Figure 6. Fluorescence emission spectra of thin-films (TFa), (TFb), (TFc), (TFd) on day 1 (black line) and day 11 (red line).
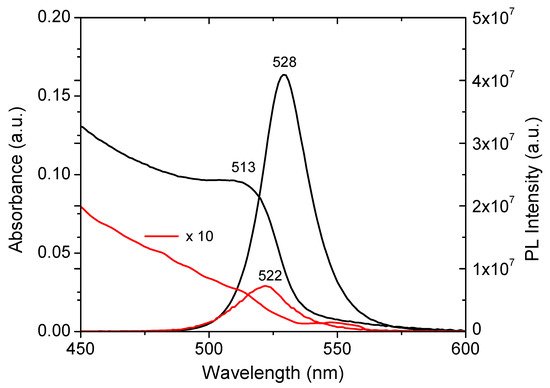
Figure 7. UV-Vis absorption and fluorescence emission spectra of TFc (red line) and TFd (black line), after 2 months.
Figure 8. UV-Vis absorption spectra of thin- films (TFa), (TFb), (TFc), and (TFd) on day 1 (black line) and day 11 (red line).
In the case of sample TFa, the UV-Vis absorption spectrum exhibits an excitonic peak at λ = 505 nm and an emission peak at 510 nm, both peaks disappeared at the second measurement (11th day). The same behavior was observed for the TFb sample, but although the excitonic absorption at ~516 nm disappeared, on the 11th day there was still a significant emission signal (Figure 6 and Figure 8). The excitonic peaks of the samples TFc and TFd in both, absorbance and emission spectra, remain strong even after two years (Figure 7) and can be observed even with naked eye, indicating a strong stability in the matrix.