The modern cultivated wheat has passed a long evolution involving origin of wild emmer (WEM), development of cultivated emmer, formation of spelt wheat and finally establishment of modern bread wheat and durum wheat. During this evolutionary process, rapid alterations and sporadic changes in wheat genome took place, due to hybridization, polyploidization, domestication, and mutation. This has resulted in some modifications and a high level of gene loss. As a result, the modern cultivated wheat does not contain all genes of their progenitors. These lost genes are novel for modern wheat improvement. Exploring wild progenitor for genetic variation of important traits is directly beneficial for wheat breeding. WEM wheat (Triticum dicoccoides) is a great genetic resource with huge diversity for traits. Few genes and quantitative trait loci (QTL) for agronomic, quantitative, biotic and abiotic stress-related traits have already been mapped from WEM. This resource can be utilized for modern wheat improvement by integrating identified genes or QTLs through breeding.
1. Introduction
With the beginning of agriculture in the Neolithic period, plants having symbiotic relation with human experienced evolutionary process which ultimately promoted human cultural development and human civilization [1]. Some of those (rice, wheat and maize) are now considered staple foods and feed a large proportion of the world’s population. Thus, a crop’s evolutionary process can be used as genetic and ecological models to evolutionary biologists for studying human–plant interactions [2]. A better understanding about the origin of crops, which remained unchanged in ploidy level during domestication from wild ancestors (such as rice and maize), has already been obtained through the advancement of modern molecular biology [3]. For many other crops, the origin, domestication, and diversification of many genes are largely unexplored. The evolution of wheat went through a long and multiple processes, including natural hybridization, polyploidization, domestication, and mutation that took place for more than 300,000 years, making it be a distinct model plant for evolutionary study [1].
At the early stage of evolution, it was difficult for new species to survive as a combination of different genome enveloped within one nucleus and followed by chromosome doubling resulted in severe genetic stress [4,5]. To cope up with stress, they had to face several challenges, such as rapid differentiation of homologous chromosomes for preventing inter-genomic pairing or securing intra-genomic pairing at meiosis and arranging inter-genomic genetic expression for harmonic coexistence [6]. These challenges are meet up through immediate genomic changes, including chromosome re-patterning, chromatin re-modelling, and molecular alteration [5,7]. Additionally, numerous morphological and physiological changes occurred during domestication which termed as ‘domestication syndrome’, including changes in seed dispersal mode, in plant architecture, increase in kernel size, loss of seed dormancy and change in nutrient content [8]. Even some genes get lost forever during evolution. Natural wheat and related allopolyploids have 2–10% less DNA than the sum of their parents which indicates elimination of DNA during evolution [9,10]. Another reason behind rapid alteration and genomic change is using different breeding method extensively. Particularly after World War II, the intensive breeding program was performed, focusing mainly on high yield. As a result, the gene pool had been narrowed down gradually, due to the enormous erosion of indigenous genetic resources [11].
For wheat improvement, adaptive genetic resources of wild progenitors and relatives can be utilized as they have enriched diversity and many beneficial traits. Exploring wild progenitor, such as wild emmer (WEM) wheat will be useful for wheat breeding and to observe wheat evolutionary changes. WEM was one of the basic plants in Neolithic agriculture, domestication of which was a key factor for the initiation of agriculture [12,13]. Landraces of WEM have huge gene pool that consists of a rich diversity for many important agronomic, qualitative biotic stress, and abiotic stress-related traits [11,14,15,16]. Many of these genes did not enter hexaploidy wheat, thus, are considered as lost genes through evolution. This manuscript will focus on gene flow, and dynamics through genomic and morphological changes occurred during the wheat evolutionary process and different approaches recovering the lost genes in WEM for modern wheat improvement.
The evolution of wheat went through a long and multiple processes, including natural hybridization, polyploidization, domestication, and mutation that took place for more than 300,000 years, making it be a distinct model plant for evolutionary study [1]. At the early stage of evolution, it was difficult for new species to survive as a combination of different genome enveloped within one nucleus and followed by chromosome doubling resulted in severe genetic stress [4,5]. To cope up with stress, they had to face several challenges, such as rapid differentiation of homologous chromosomes for preventing inter-genomic pairing or securing intra-genomic pairing at meiosis and arranging inter-genomic genetic expression for harmonic coexistence [6]. These challenges are meet up through immediate genomic changes, including chromosome re-patterning, chromatin re-modelling, and molecular alteration [5,7]. Additionally, numerous morphological and physiological changes occurred during domestication which termed as ‘domestication syndrome’, including changes in seed dispersal mode, in plant architecture, increase in kernel size, loss of seed dormancy and change in nutrient content [8]. Even some genes get lost forever during evolution. Natural wheat and related allopolyploids have 2–10% less DNA than the sum of their parents which indicates elimination of DNA during evolution [9,10].
Evolution of Wheat
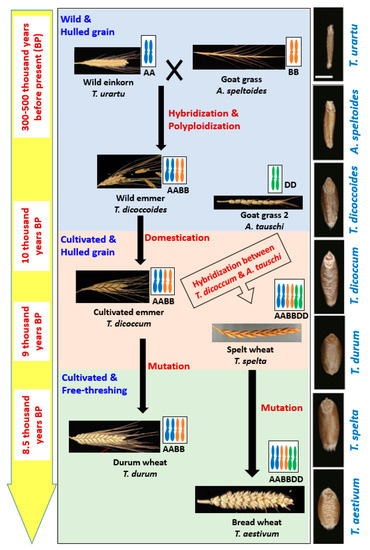
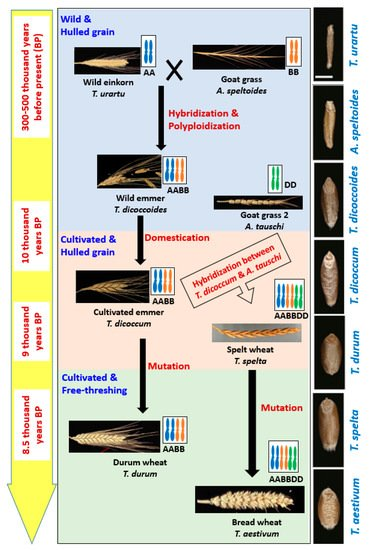
Wheat belongs to the genus Triticum which includes six species: Triticum monococcum (AA); Triticum urartu (AA); Triticum turgidum L. (AABB); Triticum timopheevii (AAGG); Triticum aestivum (AABBDD); and Triticum zhukovskyi (AAAAGG), which can be grouped into three categories: Monococcum (2x), Dicoccoidiea (4x) and Triticum (6x). The reason behind these diversified species is an evolutionary process which is truly a very complex that started at prehistoric Stone Age [2,17] (Figure 1).
2. Evolution of Wheat
Wheat belongs to the genus Triticum which includes six species: Triticum monococcum (AA); Triticum urartu Tumanian ex Gandilyan (AA); Triticum turgidum L. (AABB); Triticum timopheevii Zhuk. (AAGG); Triticum aestivum (AABBDD); and Triticum zhukovskyi Menabde and Ericz. (AAAAGG), which can be grouped into three categories: (i) Monococcum (2n = 2x), (ii) Dicoccoidiea (2n = 4x) and (iii) Triticum (2n = 6x). The reason behind these diversified species is an evolutionary process which is truly a very complex and long process that started at prehistoric Stone Age [2,17] ().
Figure 1. The central flow chart shows the evolution of wheat through hybridization, allopolyploidization, domestication and mutation along with modification in spike size and spike threshability. Left side yellow colored bar indicates the approximate time of those events happened, and right side black colored bar shows the gradual changes in grain size and shape during evolution.
WEM wheat (Triticum dicoccoides) was produced through hybridization between wild diploid wheat (T. urartu, 2n = 2x = 14, genome AA) and Goat Grass 1 (Aegilops speltoides, 2n = 2x = 14, genome BB) around 0.3–0.5 million years ago [17,18,19]. Two probable ways of developing WEM were: (i) Interspecific hybridization and then chromosome doubling in the sterile hybrid and (ii) crossing of unreduced parental gametes forming tetraploid wheat [10,20]. Cultivated emmer wheat (T. turgidum spp. Dicoccum) evolved gradually through subconscious selection from WEM by ancient people, particularly by hunter-gatherers, around 10,000 years ago in the Fertile Crescent region. The oldest evidence of cultivated emmer was observed in Tell Aswad, Syria, around 9500 years ago [2]. Moreover, some other evidence was also found in several other pre-pottery Neolithic sites in the Fertile Crescent region [2]. This region (Fertile Crescent) still has some WEM that can be divided into two groups, southern (grown in Israel, Lebanon, Palestine, and southwestern Syria) population and northern (grown in Iraq, Iran and Turkey) population [21]. Two ideas were found describing domestication of WEM: (a) Domestication was started in the northern region of the fertile crescent and instant spread to the south or vice-versa, (b) domestication occurred in both northern and southern part independently [22]. However, later, some other archaeological evidence strongly suggested independent domestication and cultivation of WEM in multiple sites [2].
Domesticated emmer hybridized spontaneously later with another wild genotype called Goat Grass 2 (Aegilops tauschii, 2n = 2x = 14, genome DD) and produced hexaploid spelt (Triticum spelta, 2n = 6x = 48, genome AABBDD) wheat around 9000 years ago [23,24]. Both cultivated emmer and spelt wheat were characterized with hulled grain, i.e., spikelet as the threshing product ().
Free-threshing durum (Triticum durum, 2n = 4x = 28, genome AABB) and bread wheat (T. aestivum, 2n = 6x = 42, genome AABBDD) were originated from enclosed cultivated emmer and spelt wheat, respectively, around 8500 years ago through natural mutation [2]. Clearly, emmer wheat played the central role of wheat evolution. Different opinions also found in the literature which explained the origin of bread wheat as a crossing product between (i) the hulled cultivated emmer (T. dicoccum), (ii) free-threshing the T. durum, or (iii) the free-threshing Triticum parvicoccum with the Ae. Tauschii [23,24]. However, the crossing took place probably 9000 years ago in south or west of the Caspian Sea, just after spreading of emmer wheat cultivation from Fertile Crescent into the natural habitat of Ae tauschii [25,26]. These studies further strengthened the central role status of emmer wheat in the evolutionary process.
The central flow chart shows the evolution of wheat along with modification in spike size and spike threshability. Left side yellow colored bar indicates the approximate time of those events happened, and right side black colored bar shows the gradual changes in grain size and shape during evolution.
Wild emmer (WEM, T. dicoccoides) was produced through hybridization between wild einkorn and Goat Grass 1 [17,18,19]. Cultivated emmer (T. turgidum spp. Dicoccum) evolved gradually through subconscious selection from WEM by ancient people, particularly by hunter-gatherers in the Fertile Crescent region. Two ideas were found describing domestication of WEM: (a) Domestication was started in the northern region of the fertile crescent and instant spread to the south or vice-versa, (b) domestication occurred in both northern and southern part independently [22]. However, later, some other archaeological evidence strongly suggested independent domestication and cultivation of WEM in multiple sites [2]. Domesticated emmer hybridized spontaneously later with another wild genotype called Goat Grass 2 and produced hexaploid spelt wheat [23,24]. Both cultivated emmer and spelt wheat were characterized with hulled grain. Free-threshing durum and bread wheat were originated from enclosed cultivated emmer and spelt wheat respectively through natural mutation [2].
Genomic Changes through Domestication
According to the history of wheat evolution, only wild einkorn and WEM wheats went through the early domestication selection [1]. T. monococcum (2x) was domesticated from the wild progenitor species T. boeoticum in the Fertile Crescent and has never been involved in the evolution of hexaploid bread wheat or tetraploid durum wheat. The wild diploid T. urartu (AA) was the progenitor of hexaploid wheat and played an essential role in wheat evolution [28]. Substantial genetic erosion occurred through the domestication process of wheat and that erosion was further reinforced during modern breeding processes [32,33,34]. Consequently, loss of diversity, selective sweeps and adaptive diversification have occurred that caused considerable genetic modification [27]. For example, nucleotide diversity at 21 gene loci was analyzed in wild, domesticated, cultivated durum and bread wheats, and revealed that diversity was reduced in cultivated forms during domestication by 69% in bread wheat and 84% in durum wheat [35].
At the very beginning of the domestication process, the major domestication trait was the seed dispersal mode [8]. Certainly plants with reduced spikelet shattering at maturity had been domesticated, which was considered as a key feature in preventing natural yield losses [8]. In addition to the yield, the other major domestication-related traits includes glume tenacity, spike compactness and flowering time (Table 1).
Table 1: Traits and gene transformed due to domestication
|
Trait
|
Transformation due to Domestication
|
Gene name
|
|
Brittle rachis [42,43]
|
Brittle rachis to non-brittleness
|
brittle rachis
(Br1, Br2 and Br3)
|
|
Glume tenacity [43,44]
|
Tough glumes to soft glumes
|
tough glumes (Tg1)
soft glumes (sog)
|
|
Spike compactness [1]
|
Non threshing or difficult threshing to free-threshing
|
threshability gene
(Q gene)
|
|
Seed shape and size/weight [40,45,46]
|
Increase in seed size and weight.
Long and thin grains to wider and shorter grain.
|
grain size (GS3)
grain weight (GW2)
seed width (SW5)
|
|
Flowering time [1,40,46]
|
Domestication involved selection of spring wheat that lack of vernalization and specific photoperiod requirement.
|
An allele on 5A of wild emmer (similar to VRN1 and at collinear position with Ppd)
|
3. Changes in the Wheat Genome during Evolution
3.1. Genomic Changes through Domestication
Generally, domestication is a selection process that provides the increased adaptation and economic viability of the plants to be cultivated in a particular environmental condition [27]. It is assumed that the first domestication of crop species started by humans 10,000 years ago [22,28]. The initial selection of wild plants as potential crops was the first step in the foundation of agriculture. However, plant selection under domestication is being continued since the Neolithic period through to plant breeding of today [29]. Through the domestication process, plants go through a suite of complex morphological, physiological, and genetic changes [30].
According to the history of wheat evolution, only wild einkorn and WEM wheats went through the early domestication selection [1]. Einkorn, T. monococcum is a diploid wheat, which was domesticated from the wild progenitor species T. boeoticum in the Fertile Crescent. Later on, it was gradually replaced by tetraploid and hexaploid wheats during the last 5000 years, approximately. Einkorn has never been involved in the evolution of hexaploid bread wheat or tetraploid durum wheat. The wild diploid Triticum species, which was the progenitor of hexaploid wheat and played an essential role in wheat evolution in T. urartu (AA) [28]. The tetraploid wheat species T. dicoccoides known as WEM naturally had been grown all over the Fertile Crescent. The early wheat growers domesticated WEM, and thus, cultivated emmer (T. dicoccum, AABB) was introduced. For a millennium or more since its domestication, emmer wheat was still growing with WEM in a complex cropping system in many Levantine sites. Thus, the genes (for example, non-brittleness gene) were transferred through spontaneous and uncontrolled hybridizations. As a result, the domesticated emmer wheat has appeared as polymorphic populations [22].
Generally, domestication aims with the elimination of undesired or even deleterious alleles, but almost in every case it also reflects an erosion of alleles valuable for plant improvement and future demands of producers and consumers [31]. It has been well documented that substantial genetic erosion occurred through the domestication process of wheat and that erosion was further reinforced during modern breeding processes [32,33,34]. Consequently, loss of diversity, selective sweeps and adaptive diversification have occurred that caused considerable genetic modification [27]. The development in molecular marker and quantitative trait loci (QTL) analysis techniques enabled to characterize those genetic losses or modifications. For example, nucleotide diversity at 21 gene loci was analyzed in wild, domesticated, cultivated durum and bread wheats, and revealed that diversity was reduced in cultivated forms during domestication by 69% in bread wheat and 84% in durum wheat [35].
The most significant effect of domestication is that genetic diversity has been reduced and is being continued through the modern plant breeding. The occurrence of genetic narrow down essentially has reduced the efficiency of crop improvement [36]. Wheat domestication by the early farmers eventually resulted in landrace cultivars (LCs) adapted to specific conditions of their habitats. At the advancement of modern plant breeding during the last century, most of the traditional LCs were continually replaced by modern wheat cultivars (MWCs) [37]. As the MWCs were bred from a few LCs they contain less genetic diversity than traditional LCs [36]. Growing wheat of such a narrow genetic diversity accelerates the risk of genetic vulnerability to the adverse condition. The risk has been further raised up due to the spontaneous mutations of a number of major insect and pathogens and the impulsive changes in environmental conditions. These might bring stresses in a new dimension that the present wheat cultivars could not cope with, and therefore, could lead to severe crop losses. During the second half of the last century number of such kind of severe crop loss had been evident. For examples, severe epidemics of shoot fly (Atherigona spp.) and kernel bunt (Tilletia indica); the outbreak of the southern corn leaf blight in the 1970s, and more recently, the outbreak of wheat blast in Bangladesh and northern India [36,38].
The genetic basis of the domestication syndrome in wheat has been extensively studied which revealed that the loss of genetic diversity in spring bread wheat occurred during (i) its domestication, (ii) the change from traditional landrace cultivars (LCs) to modern breeding varieties, and (iii) 50 years of international breeding [36]. Considerable loss of genetic diversity was observed at the early periodic domestication, and during the time of LCs to the elite breeding germplasm. It has also been evident that wheat’s genetic diversity was narrowed down more robustly during the time between 1950 and 1989. However, genetic diversity showed an uprising trend starting from 1990 indicating that breeders have experienced the consequence of narrowing down genetic diversity in the modern breeding and subsequently started to increase the genetic diversity through the introgression of novel materials. The LCs and T. tauschii contain numerous unique alleles that were absent in modern spring bread wheat cultivars [36].
It has been considered that, at the very beginning of the domestication process, the major domestication trait was the seed dispersal mode [8]. Certainly plants with reduced spikelet shattering at maturity had been domesticated, which was considered as a key feature in preventing natural yield losses [8]. In addition to the yield, the other major domestication-related traits include glume reduction (easier threshing), plant architecture (plant height, tiller numbers etc.), ear and kernel size, seed dormancy [39]. Later on, with the improvement of grain analytical process, the grain protein and mineral concentrations, as well as carbohydrate content, also became major selection attributes (). Domestication has genetically not only transformed the brittle rachis, tenacious glume and non-free threshability, but also modified numbers of other traits [8]. Meanwhile, breeding and selection had a different impact on different wheat genomes. For example, a greater number of genes related to those domestication traits are found on the A and B genomes [40]. Differential loss has been found that supports greater gene loss in the A and B genomes compared with the D [41].
TGenomic Chablnge 1s through Polyploidization
During the polyploidization of wheat, rapid alteration and several genomic changes occurred in nature. Such phenomena can be divided into two groups: Revolutionary changes, and evolutionary changes [10]. These changes can be of various types, including the elimination of both low-copy and high-copy DNA sequences, intergenomic disruption of DNA sequences, DNA methylation, deletion of rRNA, gene loss, suppression or activation of gene, chromatin modification and remodeling, heterochromatinization, sub-functionalization, and neo-functionalization [51]. Besides, hybridizations that occurred during the evolution of wheat also resulted in some significant genetic changes. For examples, in the crossing product of Aegilops sharonensis and Aegilops umbellulate, 14% loci from Ae. sharonensis and 0.5% loci from Ae. umbellulate were lost [52].
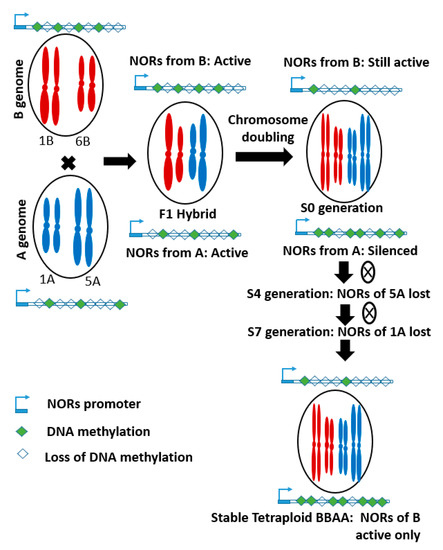
However, it is evident that evolution results in several genomic changes. For example: Nucleolus organizing regions (NORs) is present on different chromosomes (1A, 5A, 1B, 6B, and 5D) of diploid wheat [53]. Its activity is associated with the size of the intergenic regulatory region and the status of cytosine methylation [54,55]. However, NORs from the A genome are largely lost during the evolution of synthetic tetraploid wheat, due to asymmetric transcription and epigenetic modifications during polyploidization. In hybrids, NORs from both parents were expressed. After chromosome doubling, it became silenced in one parent (A genome), due to increased DNA methylation. In stable synthetic tetraploid wheat, rDNA from only the B genome was present. In the case of bread wheat, the rDNA loci form both A and D genomes were largely eliminated during evolution [7].
Chromosome-specific sequences (CSSs) occur in only one homologous chromosome pair, i.e., 1A and 1A. These types of sequences were present in all diploid species. However, after polyploidization, CSSs from one genome were eliminated immediately or after some generations. As a consequence, in hexaploid or tetraploid, these sequences occur only in one homologous pair but absent from the homeologous chromosome [57]. Meanwhile, allopolyploidization results in rapid non-random deletion of specific non-coding, low-copy and high-copy DNA [58]. Again, sometimes some genes of the A and B genomes get suppressed upon adding of D genome which is known as intergenomic suppression [10]. This is called intergenomic suppression. For example, the rust resistance gene(s) present in the A or B genome was suppressed by a gene present in the long arm of chromosome 7D [59].
3.3.
Important traits were considered for domestication and the responsible genes or QTLs with their location.
Reduction in diversity caused by intensive selection can be counterbalanced by introgression of novel germplasm. The best strategy for wheat improvement is to utilize the adaptive genetic resources of the wild progenitors, wild emmer (WEM, T. dicoccoides) and other wheat relatives [15,34,49,50].
3.2. Genomic Changes through Polyploidization
During the polyploidization of wheat, rapid alteration and several genomic changes occurred in nature. Such phenomena can be divided into two groups: (i) Revolutionary changes, and (ii) evolutionary changes. Revolutionary changes took place rapidly, during or just immediate after allopolyploidization and within a few generations, whereas evolutionary changes happened throughout the evolutionary lane for hundreds to thousands of generations and accelerated by polyploidy [10]. These changes can be of various types, including the elimination of both low-copy and high-copy DNA sequences, intergenomic disruption of DNA sequences, DNA methylation, deletion of rRNA, gene loss, suppression or activation of gene, chromatin modification and remodeling, heterochromatinization, sub-functionalization, and neo-functionalization [51]. These changes are directly or indirectly influenced by allopolyploidization. Besides, hybridizations that occurred during the evolution of wheat also resulted in some significant genetic changes as this is a very common outcome of the process. For examples, in the crossing product (hybrid) of Aegilops sharonensis and Aegilops umbellulate, 14% loci from Ae. Sharonensis and 0.5% loci from Ae. Umbellulate were lost; whereas, in the case of a cross between Ae. Sharonensis and T. monococcum, many sequences from T. monococcum were doubled in hybrid compared to another parent [52]. However, it is evident that evolution results in several genomic changes. Some examples are given below in more details.
Nucleolus organizing regions (NORs), also named as ribosomal DNA (rDNA) loci are present on different chromosomes (1A, 5A, 1B, 6B, and 5D) of diploid wheat [53]. This gene is composed of long tandem repeats that clustered on the chromosome and translated into important components of the chromosome [7]. Its activity is associated with the size of the intergenic regulatory region and the status of cytosine methylation [54,55]. However, NORs from the A genome are largely lost during the evolution of synthetic tetraploid wheat, due to asymmetric transcription and epigenetic modifications during polyploidization (). In hybrids, NORs from both parents were expressed. However, after chromosome doubling, it became silenced in one parent (A genome), due to increased DNA methylation. In this process, a pair of NOR on the 5A chromosome were deleted first, the gradual elimination of another pair from the 1A chromosome, resulted in complete loss of NORs from the A genome by S7 generation. Therefore, in stable synthetic tetraploid wheat, rDNA from only the B genome was present. In the case of bread wheat, the rDNA loci form both A and D genomes were largely eliminated during evolution [7]. Additionally, genome wide transcription analysis revealed that gene expression in synthetic bread wheat is parentally dominant and only one of the parental genomes determines morphological traits and ecological adaptations [46,51,54,56].
F Genomic Changurees through Natural Mutation 2. Schematic diagram showing the loss of Nucleolus organizing regions (NORs) from A genome, due to increased DNA methylation during the evolution process. S4 and S7 mean fourth and seventh generation of selfing (adopted from Guo and Han (2014) [7]).
Chromosome-specific sequences (CSSs) occur in only one homologous chromosome pair, i.e., 1A and 1A. These types of sequences were present in all diploid species. However, after polyploidization, CSSs from one genome were eliminated immediately or after some generations. As a consequence, in hexaploid or tetraploid, these sequences occur only in one homologous pair but absent from the homeologous chromosome [57]. Meanwhile, allopolyploidization results in rapid non-random deletion of specific non-coding, low-copy and high-copy DNA [58]. Again, sometimes some genes of the A and B genomes get suppressed upon adding of D genome. As results, they expressed in tetraploid AABB genome, but not in hexaploid AABBDD genome [10]. This is called intergenomic suppression. For example, the rust resistance gene(s) present in the A or B genome was suppressed by a gene present in the long arm of chromosome 7D [59].
3.3. Genomic Changes through Natural Mutation
Through the centuries, natural mutation resulted in significant changes in the genomic structures of wheat, which contributed substantially in the genetic evolutionary process of wheat. In general, mutation generated new alleles, while recombination created novel allele combinations. Accumulation of new mutations in older polyploid species, such as WEM, results in increased diversity and more uniform distribution across the genome [60]. For example, Genetic studies revealed that two recessive alleles at two major loci (Br-A1 and Br-B1) controlling non-brittle rachis raised through mutation during domestication [61]. One of the most important genomic changes is the evolution of free-threshing wheat as a result of several major and minor mutation events. A single major gene Q on chromosome 5AL is responsible for free-threshing of modern bread and durum wheat, whereas the recessive q allele is for non-free-threshing wild wheats [1]. A recent study showed that the Q allele arose from q allele through a gain of function mutation [62]. Free-threshabiliy is also related with tenacious glume (Tg) gene, because Tg inhibits the expression of Q gene. QTL correspond to the Tg gene is located on 2D and 2B chromosome. Free-threshing phenotype evolved when mutation transformed Tg into tg. Therefore free-threshing common bread wheat (QQ
Through the centuries, natural mutation resulted in significant changes in the genomic structures of wheat, which contributed substantially in the genetic evolutionary process of wheat. In general, mutation generated new alleles, while recombination created novel allele combinations. Accumulation of new mutations in older polyploid species, such as WEM, results in increased diversity and more uniform distribution across the genome [60]. For example, Genetic studies revealed that two recessive alleles at two major loci (Br-A1 and Br-B1) controlling non-brittle rachis raised through mutation during domestication [61]. One of the most important genomic changes is the evolution of free-threshing wheat as a result of several major and minor mutation events. A single major gene Q on chromosome 5AL is responsible for free-threshing of modern bread and durum wheat, whereas the recessive q allele is for non-free-threshing wild wheats [1]. A recent study showed that the Q allele arose from q allele through a gain of function mutation [62]. Free-threshabiliy is also related with tenacious glume (Tg) gene, because Tg inhibits the expression of Q gene. QTL correspond to the Tg gene is located on 2D and 2B chromosome. Free-threshing phenotype evolved when mutation transformed Tg into tg. Therefore free-threshing common bread wheat (QQ
tgtg
tgtg
) and free-threshing durum wheat (QQ
tgtg
2B) have mutant alleles at each of the important threshability loci [2,17].
) have mutant alleles at each of the important threshability loci [2,17].