Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Federica Rubbino and Version 2 by Catherine Yang.
Crohn’s Disease (CD) is a chronic inflammatory disorder in which up to 50% of patients develop fistula within 20 years after the initial diagnosis, and half of these patients suffer perianal fistulizing disease. The etiopathogenesis of CD-related perianal fistula is still unclear, and its phenotypical and molecular characteristics are even more indefinite. A better understanding would be crucial to develop targeted and more effective therapeutic strategies.
- Crohn-associated fistula
- epithelial-to-mesenchymal transition
1. Common Features of Fistulae in Crohn’s Disease
Crohn’s Disease (CD) is a chronic inflammation in which the disorder could affect any region of the gastrointestinal tract from the mouth to the anus (but with typically higher incidence on ileum and colon), and together with Ulcerative Colitis (UC) are stand for inflammatory bowel diseases (IBD). Although the precise beginning remains unknown, the starting point of CD is believed to result from the interplay between genetic susceptibility, environmental factors, and their interactions with gut microbiota [1][2][1,2]. Moreover, the interaction between these factors and the patient’s immune system is as well considered crucial for the pathogenesis of the disease (Figure 1a,b).
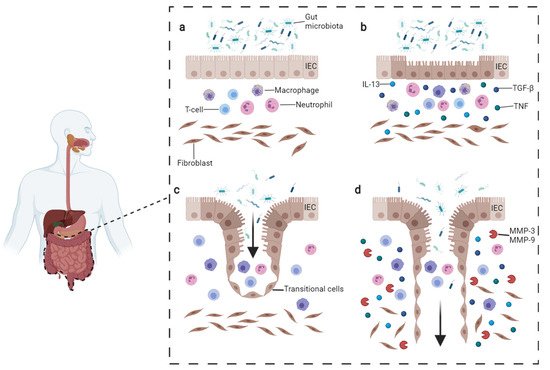
Figure 1. Pathogenesis of CD fistula. (a) Normal condition persisting in the gut. (b) In CD, the intestinal lesions can be triggered by multiple factors such as inflammatory cells reacting to microbiota or intestinal pathogens. The inflammatory infiltrate includes T-cells, B-cells, and macrophages which produce TNFα. Fibroblasts, staying underneath, produce TGF-β and IL-13. These cytokines trigger EMT and tissue remodeling. (c) During EMT, intestinal epithelial cells (IECs) lose their adhesion properties, downregulating β-catenin and E-cadherin proteins. IECs start migrating underneath in order to repair the lesion and become transitional cells (TCs). (d) TCs produce IL-13 which induces other cells to undergo EMT, penetrating deeper in lower layers. The process is facilitated by the production of metalloproteinases (MMPs) which degrade the extracellular matrix. Image adapted from Panes et al. [3][15] and drawn with BioRender.com (accessed on 23 November 2021).
CD-related fistula can be distinguished by the presence of a central fissure that invaginates deeply toward the mucosal barrier of the gut, surrounded by neutrophils, lymphocytes, and granulation tissue. The fence of the fistula canal is densely populated by T-cells CD45RO positive, macrophages CD68 positive, and B-cell CD20 positive.
In order to restore the integrity of the intestinal epithelial barrier, the defect in CLFP migration is compensated by intestinal epithelial cells (IECs) converting into transitional cells (TCs), which gain mesenchymal myofibroblast-like features through a mechanism called epithelial-to-mesenchymal transition (EMT) [4][9] (Figure 1c,d). In this complex program, IECs lose cell polarity and adherence while acquiring invasive and migratory properties (see below).
Another important factor possibly contributing to the pathogenesis of CD-related fistula is the gut microbiota. The hypothesis is indirectly supported by the fact that antibiotic therapy might be beneficial for treating fistulizing CD.
2. Signature Differences Distinguish CD-Related Fistulae from Idiopathic Ones
Although there are several recognized risk factors for Crohn’s disease [1], no environmental factors have been associated with the etiopathogenesis of CD-related fistula yet. In fact, CD-related fistula appears mostly related to genetic, microbiological, and immunological components [5][16]. Furthermore, its phenotypical and molecular characteristics are not well-characterized. Few comparative studies have allowed to identify markers able to distinguish CD fistulae from non-inflammatory bowel disease ones (also known as idiopathic or crypto-glandular). The crypto-glandular type was first classified, albeit inconclusively, by Parks in 1961 [6][17] on the basis of histological evidence of infection in anal glands. For a long time, it was thought that the persistence of the fistula depended upon inflammation, the presence of bacterial endotoxins perpetuating an inflammatory response within the lumen even when bacteria are destroyed [7][18], and upon the presence of bacterial pro-inflammatory peptidoglycan (PG) which may stimulate the secretion of interleukin-1β (IL-1β) [8][19]. The first evaluations on specific inflammatory characteristics of the fistulae were carried out by immunohistochemistry (IHC). CD fistulae diverge considerably from non-CD fistulae concerning their inflammatory composition, except for the lining epithelium. CD fistulae present high levels of infiltrated T cells CD45RO positive in the interior wall, dense infiltration of B cells CD20 positive surrounding lined up by a small group of macrophages CD68 positive. The second type has an intense infiltration of CD68 positive macrophages enclosed by CD45RO positive T lymphocytes [9][8]. Metalloproteases (MMP) and tissue inhibitors of metalloproteinases (TIMPs), which contribute to fistula formation through extracellular matrix degradation, did not show any difference between the two types of fistulae, with exception of MMP-3 and 9 that appear to be upregulated in idiopathic fistula [10][20]. Abundant expression of IL-1β, IL-8, IL-12p40 cytokines, and tumor necrosis factor (TNF)-α were found in idiopathic anal fistulae [11][21]. However, TNF-α was also significantly up-regulated in the serum of CD-related perianal fistula patients [12][22]. Data on inflammation and EMT were confirmed also in non-CD fistulae through real-time PCR (RT-PCR) and Western blot analysis beside IHC. The inflammation was evaluated by the expression of IL-8 and IL-1β that were respectively more expressed in the proximal part than in the distal one and vice versa for the second marker [13][14][24,25]. The expression of markers such as TGF-β, Vimentin, ZEB1, SNAIL which were highly expressed in both proximal and distal parts, with mild E-cadherin reduction, were used for EMT evaluation [14][25]. Antimicrobial peptides, that are controlled in response to a bacterial antigens provocation or the presence of inflammatory cytokines, specifically hBD2 and hBD3 together with RNase7 and psoriasin, resulted to be elevated in the distal area of non-CD fistulae [13][24]. Furthermore, flow cytometry analysis (FACS) on curettage and tissue biopsy of CD-related fistulae do not present differences in the ratio of CD161+, IL-17+IFN-γ−, IL-17−IFN-γ+ and IL-17+IFN-γ+ cells between CD4+ or CD8+ T cells. CD161+ T lymphocytes were instead more expressed [15][26]. Proteomic analysis was performed to evaluate potential dissimilarity in cytokines and phosphoprotein concentration. The phosphorylation status of 28 Receptor Tyrosine Kinases (RTKs) and 11 signaling nodes in addition to 30 cytokines and chemokines was quantified. The two types of fistulae do not substantially differ in their protein expression pattern, even though the panel of cytokines and phosphoproteins analyzed was huge [16][27]. It is plausible that metabolic changes accompanying EMT precede some of the more obvious signals of mesenchymal transition and indeed actively contribute to the activation of these indicators [17][29]. All described signatures are schematized in Figure 2.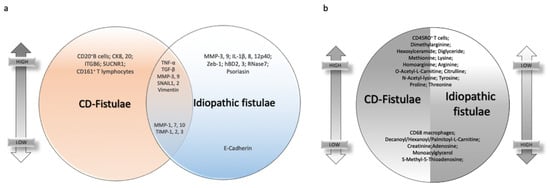
Figure 2. Common and dissimilar signatures between CD- and idiopathic fistulae. (a) Venn diagram highlighting individual or common markers expression in CD-related fistulae and idiopathic ones, on the top if they are upregulated, on the bottom if they are downregulated. (b) Differential markers expression in CD-related fistulae versus idiopathic fistulae. On the right of the circle, the expression is lower on the top of the graph and higher on the bottom. Vice versa, on the left part of the circle, the expression of the same markers is higher on the top and lower on the bottom.
3. Therapeutic Approaches to CD-Related Fistulae
Perianal fistulizing CD is a particularly challenging form of CD, presenting in up to one-third of the patients [18][53]; in a tiny percentage of cases, it can be the only manifestation of the disease and may precede by several years intestinal manifestations of CD in up to 10% of the patients [19][54]. The presence of fistulae is often associated with an aggressive form of CD, with chronic course and disappointing rates of long-lasting remission [20][55].
As a consequence, patients commonly experience a negative impact on quality of life, including intimate and social relationships and a frequent need for hospital admissions and medical observations [21][6].
The establishment of biologic therapy has dramatically improved the efficacy of medical treatment of CD fistulae compared to the previous use of traditional immunomodulators, and, at present, anti-TNFα represent the therapy of choice in these patients [22][23][56,58]. The effectiveness of biologic therapy basically depends on the capability of these drugs to reduce tissue inflammation, which is the driving mechanism for fistulae development.
Medical therapy alone has demonstrated remission rates around 60%, and its combination with surgery improves reaction, recurrence rate, as well time to recurrence [24][59].
Anyway, immunosuppression by anti-TNF agents needs to evaluate the presence of abscesses (and the possibility to resolve them by drainage and antibiotics), due to their potential septic complications.
The use of anti-TNFα has also been studied as to its local/topic injections, with the aim to potentiate the efficacy on fistula healing [25][26][27][60,61,62]. Although several reports have shown promising results, this technique has not been standardized yet, therefore it can be intended as a supportive tool in case other approaches have failed or are not available.
The use of biologics other than anti-TNFα (ustekinumab and vedolizumab) is not currently recommended as first-line therapy and should be considered only in case of contraindications to anti-TNFα [22][56].
The closure of the fistula tract can be attempted by using different techniques, either endoscopic or surgical, and materials including fibrine glue, plugs, and n-butyl-2-cyanoacrylate (Histoacryl). Among these, fibrin glue injection is the most common technique, with a good safety profile and limited costs, although penalized by limited efficacy [28][64]. The insertion of fistula plugs has also been tested; the procedure consists of the application of a bio-absorbable xenograft which should promote tissue regeneration and fistula closure. This technique has demonstrated a success rate equal to seton drainage [29][65], hence it is not recommended.
Considering endoscopic techniques, fistula closure can be achieved by clipping with either through-the-scope or over-the-scope clips [30][66]. Clipping is effective in fistula closure, being a safe and simple procedure in the hands of trained endoscopists.
The advancement flap is probably the most used among the surgical techniques. The procedure was first developed for treating cryptoglandular fistulae but is now routinely applied also in CD patients. Fistula healing is complete in about half of the patients [31][67].
Of comparable efficacy, ligation of the intersphincteric fistula tract (LIFT), which was also initially described in the treatment of cryptoglandular fistulae and has been then transferred to CD associated fistulae [19][54]. Consisting of the opening of the intersphincteric groove, dissection, and isolation of the fistulous tract, ligation of the tract with interrupted sutures and closure of the perianal wound LIFT is a demanding procedure and should always be performed by dedicated surgeons. Compared to advanced flap procedure, LIFT has demonstrated lower incontinence rates (7.8% versus 1.6%) [32][68].
In order to preserve the sphincter functionality, the video-assisted anal fistula treatment (VAAFT) consists of the video-assisted inspection of the fistula followed by a precise cauterization of the fistula tract from the external towards the internal margin, and closure of the internal opening. Although not yet routinely applied in CD fistula surgery, this technique appears promising, especially for the benefits of sphincteric preservation [33][69]. Similar to VAAFT, the fistula laser closure technique applies laser instead of electrocautery and is not performed under direct vision. This technique has similar efficacy to VAAFT but shorter learning curves [34][70].
Overall, surgery for CD fistulae should always be performed by expert operators in high-flow centers after adequate study of the clinical case. Local availability and expertise should guide the choice of the technique.
The limited success rate of combined medical and surgical therapy, although being slightly improved, has promoted the research of novel methods. One of the most promising is the injection in the fistula tract of mesenchymal stem cells (MSCs), aimed at tissue regeneration using a minimally invasive procedure [35][71].
MSCs are non-hematopoietic multipotent cells, which can be set apart from connective tissues, like adipose tissue, and from the bone marrow. These cells have been studied in fistula treatment due to their immunomodulatory, immunosuppressive, and regenerative properties [36][72]. Their use as treatment of CD fistulae has been described by Panés et al. in the ADMIRE trial [37][73] with promising results in terms of efficacy and safety, which paved the way to their entrance also in the last ECCO guidelines [38][57].
The progressive in-depth analysis on the pathogenetic mechanisms of CD fistulae has allowed hypothesizing new promising therapeutic tools, such as anti-MMP antibodies. Studies about anti-MMP drugs start from the assumption that MMP-9, a type IV collagenase, has a central role in tissue remodeling and is upregulated in crypt abscesses and around fistulae [39][74]. A study by Fontani et al. [40][75] showed in vitro that N-acetylcysteine and curcumin were able to downregulate MMP-3 in high oxidative state conditions, and specifically in TNFα stimulated cells, suggesting that such antioxidants may have a therapeutic use for the prevention and treatment of fistulae in the gut of CD patients.
Another study by Goffin et al. [41] was conducted in vitro from human specimens and in mice xenograft, confirming in the patients affected by penetrating CD the upregulation of MMP-9, and showing in mice a protective effect of anti-MMP antibodies with respect to intestinal fibrosis. Albeit in literature only animal- and in vitro studies are available, the future application of such molecules could revolutionize the treatment of perianal fistulae.
The understanding of the crucial role of inflammation in fistula development has sustained the still limited but promising application of hyperbaric oxygen therapy as supportive treatment in patients affected by perianal CD [42][76]. The treatment consists of breathing 100% oxygen under increased atmospheric pressure, provoking tissue hyperoxygenation and oxidative stress which has been associated to stem cell mobilization and upregulation of growth factors and ultimately to anti-inflammatory effects. Considering its safety and limited costs if the equipment is available, this treatment appears a valid supportive method for patients with otherwise unsatisfactory healing [43][77].
