Oral diseases (ODs) are highly prevalent affecting over 3.5 billion people, particularly in low- and middle-income countries (LMICs). Saliva is often described as the "mirror of the body" and so different omics approaches such us proteomics, metabolomics and more recently, volatomics are being employed to explore the potential of this biofluid towards the non-invasive diagnosis of ODs.
- saliva
- volatile organic compounds (VOCs)
- oral diseases (ODs)
- biomarkers
1. Introduction
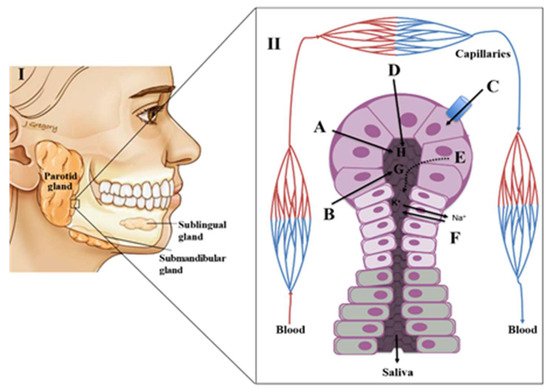
2. Physiology of Saliva
Saliva Composition and Production
3. Putative Salivary Biomarkers for Oral Diseases
3.1. Gingivitis and Periodontal Disease
3.2. Dental Caries
3.3. Oral Cancer
3.4. Oral Potentially Malignant Disorders (OPMD)
3.5. Burning Mouth Syndrome
3.6. Recurrent Aphthous Ulceration (RAS)
4. Salivary Volatomics
Condition | Putative Volatile Biomarker | Metabolic Context | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Periodontal disease | |||||||||||
pyridine and three methylpyridine isomers (picolines) | detected in patients but not in controls |
[60] |
[78] |
||||||||
hydrogen sulphide | oral bacteria infection | ||||||||||
methyl mercaptan | oral bacteria infection | ||||||||||
Halitosis | |||||||||||
dimethyl disulphide | oral bacterial infection |
[60] |
[78] |
||||||||
dimethyl disulphide, carbon disulphide, VSCs | drug-related metabolism |
[73] |
[83] |
||||||||
VSCs | microbial degradation products of the sulphur-containing amino acids cysteine, cystine and methionine |
[60] |
[78] |
||||||||
VSCs | augmented levels detected upon anxiety challenge |
[74] |
[84] |
||||||||
VSCs, aliphatic amines, branched chain fatty acids, indole and phenol | oral bacteria metabolism |
[59] |
[77] |
||||||||
Putrescine, cadaverine, histamine, tyramine, indole, skatole, mercaptans and sulphides | microbial metabolism of proteinaceous substrates |
[75] |
[85] |
||||||||
2,3-butanedione; 2,3-pentanedione; | Phenol; pyrrole; indole and dimethyl disulphide | bacterial | metabolism of lipids and carbohydrates |
[62] |
[80] |
||||||
indole and skatole | bacterial fermentation products of tryptophan |
[60] |
[78] |
||||||||
phenol and p-cresol | bacterial putrefaction metabolites of phenolic amino acids | ||||||||||
Oral candidiasis | |||||||||||
3-methyl-2-butanone and styrene | Candida albicans infection |
[70] |
[86] |
||||||||
p-xylene, 2-octanone, 2-heptanone and n-butyl acetate | Candida krusei infection | ||||||||||
Oral cancer | |||||||||||
1,4-dichlorobenzene; 1,2-decanediol; 2,5-di-tert-butylphenol and E-3-decen-2-ol | identified in head and neck cancer cohorts |
[76] |
[87] |
||||||||
Dietary origin | |||||||||||
2-heptanone, benzaldehyde, dodecanal, 2-butyl-1-octanol, allyl isothiocyanate | examples of ketones, aldehydes, alcohols, esters and VSCs obtained from our diet |
[62] |
[80] |
||||||||
Oxidative stress | |||||||||||
hexanal and nonanal | general markers for oxidative damage (endogenously produced from | membrane lipid oxidation) |
[62] |
[80] |
|||||||
Environmental contaminants (air pollutants) | |||||||||||
long-chain alkane derivatives (hexane, octane and undecane); aromatic compounds (as benzene, toluene, xylenes and styrene) | common air pollutants found in saliva |
[60] |
[78] |
