According to the National Institutes of Health (NIH, USA), biomarkers are objectively measured indicators of physiologic processes, pathologic processes or pharmacologic responses to a therapeutic intervention
[1][2][6,7]. In MS, they can be subdivided into diagnostic (help to differentiate between different diseases, e.g., anti-aquaporin-4 antibodies, oligoclonal bands, etc.), prognostic (enable physicians to estimate how a disease might develop once it has been diagnosed, e.g., neurofilaments, oligoclonal bands, etc.), predictive (predict the treatment response and thus help to decide which patient is most likely to benefit from a certain treatment), disease activity (measure the inflammatory/neurodegenerative components of the disease, e.g., MRI, clinical parameters, etc.) and treatment response (responders versus non-responders of a certain treatment) biomarkers
[1][6]. Especially with the focus on personalized medicine in pwMS, treatment response biomarkers can enable neurologists to differentiate patients regarding efficacy (e.g., neurofilament light chains, neutralizing antibodies against interferon-ß or natalizumab) or potential side effects (e.g., anti-varicella zoster virus antibodies, anti-John Cunningham virus antibodies) of a certain treatment
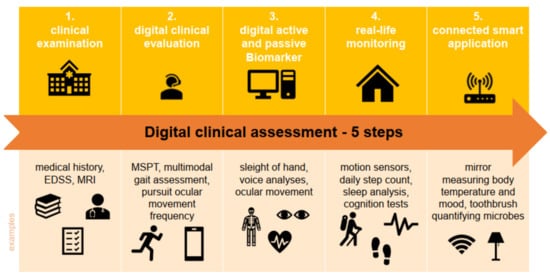
[1][6]. The collection of such data is crucial to adapt the treatment of each patient individually to his/her results. However, it is also time-consuming if these data have to be gathered by physicians or other healthcare staff. With the increasing digitalization of healthcare, medicine now gains access to a new type of biomarker. So-called digital biomarkers enable the translation of up-to-date new data sources into informative, actionable knowledge. They can be used by healthcare professionals (HCPs) by implementing digital devices in their assessment (e.g., MRI, optical coherence tomography (OCT) and tablet-based neurostatus); they also enable data collection directly from the patient. They can collect such data directly as part of disease management on a regular basis, and thus ensure good monitoring and a prompt reaction to the progression of MS and the worsening of symptoms. Digital biomarkers mean objective, quantifiable physiological and behavioral data that are measured and collected by digital devices. The data collected by, e.g., portables, wearables, implantables or digestibles are typically used to generate, influence and/or predict health-related outcomes, and thus represent deep digital phenotyping, collecting clinically meaningful and objective digital data
[3][8]. As digital technologies are usually less expensive than the process of collecting these data face to face, and as some of these data can be collected even without patients being actively involved (passive monitoring, e.g., by the use of wearables) data can also be collected more frequently and longitudinally. Health-related outcomes can vary, from explaining health and disease states, predicting drug responses or influencing health behaviors. In addition to this rather strict definition of digital biomarkers, digitalization in medicine also includes patient-reported measures (e.g., survey data), genetic information and other data that now can be collected by digital infrastructure. These data can complement the mentioned digital biomarkers, creating a digital multidimensional dataset.