Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Tarik Smani Hajami.
Ucn is a mammalian peptide member of the corticotropin-releasing factor (CRF) family. Three isoforms of Ucn have been described so far, Ucn1, Ucn2, and Ucn3, which differ in terms of their structure, expression, and affinity to CRF receptors.
- urocortin
- adverse cardiac remodeling
- cardioprotection
1. Introduction
Despite the progress in cardiovascular disease treatment, the healthcare burden of ischemic heart failure (HF) is increasing worldwide [1]. Heart ischemia is originated by the critical obstruction of coronary arteries, leading to an imbalance between the consumption and supply of nutrients in the affected area in the heart. Ischemia causes cells lesions of different degrees depending on the extent of blood flow reduction and the length of the ischemic period, which influence the reduction in pH, ATP, and creatine phosphate, as well as increased levels of intracellular Na+ and Ca2+ concentrations, enlarged cell volume, and intracellular membranes disruption [2]. Treatment for myocardial ischemia involves the prompt and timely recovery of blood flow, which is known as myocardial reperfusion or revascularization and is necessary to save oxygen-deficient tissue (for a review, see [3,4][3][4]). Paradoxically, reperfusion causes additional injuries due to metabolites reaction with oxygen giving rise to reactive oxygen species (ROS) known as oxygen paradox [5]; stress of sarcoplasmic reticulum (SR), producing the accumulation of secondary metabolites and bad protein products; capillary non-reflow that leads to a worse local reperfusion [6,7,8][6][7][8]; and the mishandling of the intracellular Ca2+ concentration ([Ca2+]i) [9]. The pathophysiological value of [Ca2+]i homeostasis is well-recognized. Actually, one of the critical factors involved in I/R syndrome is the cytoplasmic Ca2+ overload produced by the abnormal Ca2+ homeostasis. Isolated cardiac myocytes after I/R presents an increase in diastolic [Ca2+]i, a decrease in the systolic [Ca2+]i transients, and a reduction of SR Ca2+ load, which correlate with a decrease in the activity of Na+/Ca2+ exchanger (NCX) [9,10][9][10]. All these events culminate in the mitochondrial permeability transition pore (MPTP) opening, apoptosis, and cell death [5] as illustrated in Figure 1. This phenomenon is widely known as I/R syndrome [11,12][11][12].
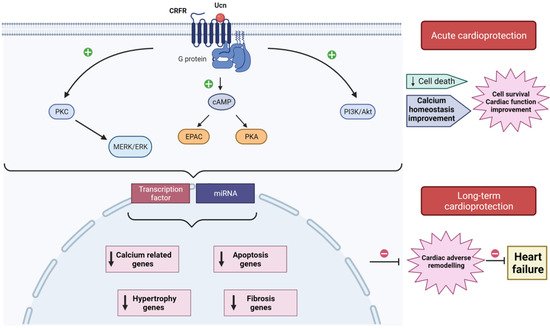
Figure 1. Schematic model illustrating acute and long-term cardioprotection afforded by urocortin (Ucn) from I/R injuries. In heart, Ucn binds to CRFR2, which interacts with G-proteins activating different signaling pathways (PKC-MERK/ERK; cAMP-EPAC/PKA; PI3K/Akt) that acutely decrease cell death, improve Ca2+ handling, enhance cell survival, and improve cardiac function. Ucn also activates transcription factors and stimulates miRNAs, release which regulates the expression of genes related to apoptosis, fibrosis, hypertrophy, and Ca2+ homeostasis. The downregulation of these genes prevents the development of adverse cardiac remodeling, avoiding its progress toward heart failure.
Sequelae of acute myocardial infarction (AMI) are known to trigger the adverse cardiac remodeling, which is the basic mechanism underlying the progression to HF considered the end stage of various types of cardiovascular disease, including ischemic heart disease (IHD) [13]. Therefore, there is an urgent need for adjunct cardioprotection therapy to prevent the impact of I/R injuries on heart function [14,15][14][15].
In the last two decades, Urocortin (Ucn) isoforms, peptides related to stress, arose as potential therapeutic drugs to improve performances of heart in I/R and under HF [16,17][16][17].
2. Structure and Expression of Urocortin
Ucn is a mammalian peptide member of the corticotropin-releasing factor (CRF) family. Three isoforms of Ucn have been described so far, Ucn1, Ucn2, and Ucn3 [18,19,20,21][18][19][20][21], which differ in terms of their structure, expression, and affinity to CRF receptors. Ucn1 is a 40 amino acid (aa) peptide having 63% and 45% sequences identity with urotensin and CRF, respectively [18]. Ucn1 was first identified in the brain, where it is expressed mainly in the Edinger–Westphal nucleus, but it was also located in the supraoptic nucleus, lateral olive, and lateral septum [18,22,23][18][22][23] and in other brain regions [24]. The expression of Ucn1 mRNA was also detected in the atrium and the ventricle of human heart. Similarly, the presence of Ucn1 peptide was demonstrated by immunocytochemistry assay in human heart [25]. Ucn1 was also detected at the mRNA and protein levels either in rat cardiac myocytes or fibroblasts [16,26][16][26] and in other peripheral tissues such as in the gastrointestinal system [27,28][27][28], the immune system, thymus, liver, adrenal gland, placenta, skin, and skeletal muscles [29,30][29][30].
Ucn2 is formed by 38 aa showing homologies with rat and human CRF (34%) and Ucn1 (43%). Meanwhile, Ucn3 (38–41 aa) has 37–40% homology with Ucn2 [19,20,21][19][20][21]. The expression of Ucn2 and Ucn3 was first studied using PCR that revealed their high expression in colon, small intestine, muscle, stomach, thyroid, adrenal, pancreas, spleen, and heart [20]. Particularly, experimental models using rodents showed that Ucn2 is also expressed in the central nervous system and peripheral tissues, being highly detected in cardiac myocytes [31], in skeletal muscle and skin [32]. Mouse Ucn3 mRNA expression was found in areas of the brain, small intestine, and skin. Different studies also demonstrated that neurons from the hypothalamus and amygdala also express Ucn3 [24]. Using RT-PCR and immunostaining, it was demonstrated that Ucn3 is also expressed in human heart [25,33,34][25][33][34].
3. Corticotropin Releasing Factor Receptors and Signaling Pathways
Ucn isoforms have high affinity to CRF receptors CRFR1 and CRFR2, which are G-protein coupled receptors that trigger various downstream signal transduction pathways. CRFR1 is mainly expressed in the brain, while CRFR2 is highly expressed in cardiac cells and peripheral tissue [35]. CRFR2 has three variants (α, β, γ), which vary in their N-terminal extracellular domains conferring differences in their subcellular localization [36]. CRFR2α was detected in all chambers of human heart, and CRFR2β was specifically identified in the left atrium [25,37][25][37]. Ucn isoforms and receptors are expressed within the left ventricle of the human myocardium at mRNA level, including CRFR1 and CRF [38]. Of note, this study showed a downregulation of CRFR2α and upregulation of CRFR1, CRF, and Ucn3 in diseased hearts excised from patients undergoing heart transplantation. In addition, a new splicing variant of CRFR1 gene named CRFR1j apparently is more expressed in HF patients than in healthy donor [38].
Structurally, CRFR1 and CRFR2 are approximately 70% identical at the aa level, but they exhibit considerable divergence at the N-terminal extracellular domain. Hence, they display ligand selectivity between Ucn members [24]. Indeed, Ucn1 binds with similar affinity to CRFR1 and CRFR2; meanwhile, Ucn2 and Ucn3 are selective ligands for CRFR2 [19,21][19][21]. CRFR2 interacts with Gs, Gq, and Gi proteins, activating different signaling pathways [39]. Independent studies demonstrated that the stimulation of CRFR2 contributes to the activation of cAMP and protein kinase A (PKA) signaling cascade [26], exchange protein activated by cAMP (Epac), extracellular signal-regulated kinase (ERK), and protein kinase C (PKC) [40]. For instance, experiments using ex vivo Langerdorff-perfused hearts and isolated cardiac myocytes showed that Ucn through CRFR2 induced potent positive inotropic and lusitropic effects, involving Epac, PKC. and mitogen-activated protein kinases (MAPK) signaling pathways [41]. CRFR2 stimulation also induced AMP-activated protein kinase (AMPK), phosphatidylinositol 3-kinase (PI3-K), and Akt kinase activation in isolated heart muscle and in vivo in intact hearts [31,42][31][42]. By contrast, CRFR2 antagonist markedly decreased the induced phosphorylation of PKA, CREB, CaMKII, and AKT [35].
Altogether, signaling pathways activated downstream CRFR2 play a critical role in different aspects of myocardial function, which explain the many effects that Ucn isoforms have on the cardiovascular system.
4. Acute Action of Urocortin in the Cardioprotection
The three isoforms of Ucn have established protective actions against myocardial I/R since they attenuate I/R incidences and the consequent cardiac adverse remodeling. The early studies about Ucn highlighted its acute effects in Langendorff-perfused mice, rat, and sheep hearts or using isolated neonatal rat ventricular myocytes (NRVM) and adult cardiac myocytes (for a review, see [43]). The acute administration of Ucn before ischemia or at the onset of reperfusion improved cardiac hemodynamic parameters, decreased the infarct size, and attenuated apoptosis assessed by TUNEL staining, caspase activity, or lactate dehydrogenase (LDH) [44,45,46,47][44][45][46][47]. Moreover, Ucn addition before ischemia and during reperfusion, but not only during reperfusion, significantly restored ATP and creatine phosphate in heart tissue, which are high-energy molecules required to prevent cell death after injury. Accordantly, Ucn inhibited creatine phosphokinase in similar conditions [45]. Similarly, the addition of Ucn before reperfusion prevented I/R-induced diastolic Ca2+ overload and recovered completely the amplitude of [Ca2+]i transients; thus, it improved [Ca2+]i handling and improved heart contractility [9]. Interestingly, cardiac cell incubation with Ucn2 inhibited I/R-upregulation of proteins related to the store-operated Ca2+ signaling pathway, such as Orai1, TRPC5, and STIM1 [48] (Figure 1).
Interestingly, in addition to Ucn isoforms’ acute effects, increasing evidence demonstrated that they also have durable cardioprotective effects, attenuating events related with the adverse cardiac remodeling, such as the regulation of apoptotic genes and fibrosis. For instance, Ucn inhibited I/R-induced cardiac autophagy by Beclin1 downregulation through the PI3K/Akt pathway [49]. Ucn1 increased the expression of apoptotic genes CD40lg, Xiap, and BAD at mRNA and protein levels, in cells undergoing I/R through Epac2 and ERK1/2 activation [50]. Actually, this study demonstrated that Ucn1 promotes apoptosis programmed cell death to the detriment of necrosis, which will limit the impact of cardiac cell loss and posterior inflammatory processes in I/R [50]. In contrast, another study associated Ucn2 cardioprotective actions with the overexpression of the anti-apoptotic gene Bcl-2 and the downregulation of pro-apoptotic genes Bax and Bim [51]. It is not yet clear in which situation Ucn isoforms activate or inhibit apoptotic genes under I/R, which is worth deeply investigating. In addition to apoptotic genes, Ucn also stimulated the upregulation of other genes, such as cardiotrophine-1 (CT-1) [46], potassium channel Kir 6.1 [52], glucocorticoid-responsive kinase-1 (SGK1) [53], or Orai1 and TRPC5 channels [48], suggesting that the cardioprotective action of Ucn isoforms involved a wide range of signaling pathways.
More recently, two independent studies showed that Ucn1-induced cardioprotection involved microRNAs (miRNAs) dysregulation in Langendorff-perfused heart subjected to global ischemia and reperfusion [54,55][54][55]. miRNAs are small non-coding RNAs that regulate a plethora of cellular processes related to the adverse cardiac remodeling, including cardiac myocyte apoptosis, necrosis, and fibrosis [56]. In fact, under I/R, Ucn1 upregulated miR-125a-3p, miR-324-3p, and downregulated miR-139-3p. Further experiments using NRVM demonstrated that the effect of Ucn1 involved the activation of CRFR2, Epac2, and ERK1/2. Furthermore, the overexpression of miR-125a-3p, miR-324-3p, and miR-139-3p modulated the expression of different genes involved in cell death and apoptosis (BRCA1, BIM, STAT2), in cAMP and Ca2+ signaling (PDE4a, CASQ1), in cell stress (NFAT5, XBP1, MAP3K12), and in cell metabolism (CPT2, FoxO1, MTRF1, TAZ). Similarly, Ucn2 was suggested to upregulate miRNA-221 in perfused heart, which inhibited apoptotic (BIM, BMF, Ddit4, p27) and autophagic genes (LC3-II) both at mRNA and protein levels [54].
Altogether, these data demonstrated a novel role of Ucn in myocardial protection, involving post-transcriptional regulation of genes through miRNAs [55].
References
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580.
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170.
- Ribichini, F. Acute myocardial infarction: Reperfusion treatment. Heart 2002, 88, 298–305.
- Mathew, V.; Gersh, B.J. To open or not to open: That remains the question. Eur. Heart J. 2004, 25, 2177–2179.
- Bugger, H.; Pfeil, K. Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165768.
- Calderón-Sánchez, E.M.; Ávila-Medina, J.; Callejo-García, P.; Fernández-Velasco, M.; Ordóñez, A.; Smani, T. Role of Orai1 and L-type CaV1.2 channels in Endothelin-1 mediated coronary contraction under ischemia and reperfusion. Cell Calcium 2020, 86, 102157.
- Eeckhout, E.; Kern, M.J. The coronary no-reflow phenomenon: A review of mechanisms and therapies. Eur. Heart J. 2001, 22, 729–739.
- Niccoli, G.; Burzotta, F.; Galiuto, L.; Crea, F. Myocardial No-Reflow in Humans. J. Am. Coll. Cardiol. 2009, 54, 281–292.
- Golden, J.; O’Dwyer, A.M.; Conroy, R.M. Depression and anxiety in patients with hepatitis C: Prevalence, detection rates and risk factors. Gen. Hosp. Psychiatry 2005, 27, 431–438.
- Roe, A.; Frisk, M.; Louch, W. Targeting Cardiomyocyte Ca2+ Homeostasis in Heart Failure. Curr. Pharm. Des. 2014, 21, 431–448.
- Zhao, Z.-Q.; Corvera, J.S.; Halkos, M.E.; Kerendi, F.; Wang, N.-P.; Guyton, R.A.; Vinten-Johansen, J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am. J. Physiol. Circ. Physiol. 2003, 285, H579–H588.
- Hausenloy, D.; Yellon, D. Preconditioning and postconditioning: Underlying mechanisms and clinical application. Atherosclerosis 2009, 204, 334–341.
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214.
- Hausenloy, D.; Botker, H.E.; Engstrom, T.; Erlinge, D.; Heusch, G.; Ibanez, B.; Kloner, R.A.; Ovize, M.; Yellon, D.; Garcia-Dorado, D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: Trials and tribulations. Eur. Heart J. 2016, 38, 935–941.
- Heusch, G.; Rassaf, T. Left Ventricular Unloading in Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 76, 700–702.
- Brar, B.K.; Jonassen, A.K.; Stephanou, A.; Santilli, G.; Railson, J.; Knight, R.A.; Yellon, D.; Latchman, D.S. Urocortin Protects against Ischemic and Reperfusion Injury via a MAPK-dependent Pathway. J. Biol. Chem. 2000, 275, 8508–8514.
- Brar, B.K.; Jonassen, A.K.; Egorina, E.M.; Chen, A.; Negro, A.; Perrin, M.H.; ΜjøsO, D.; Latchman, D.S.; Lee, K.-F.; Vale, W. Urocortin-II and Urocortin-III Are Cardioprotective against Ischemia Reperfusion Injury: An Essential Endogenous Cardioprotective Role for Corticotropin Releasing Factor Receptor Type 2 in the Murine Heart. Endocrinology 2004, 145, 24–35.
- Vaughan, J.; Donaldson, C.J.; Bittencourt, J.; Perrin, M.H.; Lewis, K.; Sutton, S.; Chan, R.; Turnbull, A.V.; Lovejoy, D.; Rivier, C.; et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nat. Cell Biol. 1995, 378, 287–292.
- Lewis, K.; Li, C.; Perrin, M.H.; Blount, A.; Kunitake, K.; Donaldson, C.; Vaughan, J.; Reyes, T.M.; Gulyas, J.; Fischer, W.; et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 7570–7575.
- Hsu, S.Y.; Hsueh, A.J. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med. 2001, 7, 605–611.
- Reyes, T.M.; Lewis, K.; Perrin, M.H.; Kunitake, K.S.; Vaughan, J.; Arias, C.A.; Hogenesch, J.B.; Gulyas, J.; Rivier, J.; Vale, W.W.; et al. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 2843–2848.
- Kozicz, T.; Yanaihara, H.; Arimura, A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J. Comp. Neurol. 1998, 391, 1–10.
- Morin, S.; Ling, N.; Liu, X.-J.; Kahl, S.; Gehlert, D. Differential distribution of urocortin- and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neuroscience 1999, 92, 281–291.
- Hillhouse, E.W.; Grammatopoulos, D. The Molecular Mechanisms Underlying the Regulation of the Biological Activity of Corticotropin-Releasing Hormone Receptors: Implications for Physiology and Pathophysiology. Endocr. Rev. 2006, 27, 260–286.
- Kimura, Y.; Takahashi, K.; Totsune, K.; Muramatsu, Y.; Kaneko, C.; Darnel, A.D.; Suzuki, T.; Ebina, M.; Nukiwa, T.; Sasano, H. Expression of Urocortin and Corticotropin-Releasing Factor Receptor Subtypes in the Human Heart. J. Clin. Endocrinol. Metab. 2002, 87, 340–346.
- Nishikimi, T.; Miyata, A.; Horio, T.; Yoshihara, F.; Nagaya, N.; Takishita, S.; Yutani, C.; Matsuo, H.; Matsuoka, H.; Kangawa, K. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. Am. J. Physiol. Circ. Physiol. 2000, 279, H3031–H3039.
- Harada, S.; Imaki, T.; Naruse, M.; Chikada, N.; Nakajima, K.; Demura, H. Urocortin mRNA is expressed in the enteric nervous system of the rat. Neurosci. Lett. 1999, 267, 125–128.
- Muramatsu, Y.; Fukushima, K.; Iino, K.; Totsune, K.; Takahashi, K.; Suzuki, T.; Hirasawa, G.; Takeyama, J.; Ito, M.; Nose, M.; et al. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides 2000, 21, 1799–1809.
- Gutknecht, E.; Van der Linden, I.; Van Kolen, K.; Verhoeven, K.F.C.; Vauquelin, G.; Dautzenberg, F.M. Molecular Mechanisms of Corticotropin-Releasing Factor Receptor-Induced Calcium Signaling. Mol. Pharmacol. 2009, 75, 648–657.
- Asaba, K.; Makino, S.; Nishiyama, M.; Hashimoto, K. Regulation of Type-2 Corticotropin-Releasing Hormone Receptor mRNA in Rat Heart by Glucocorticoids and Urocortin. J. Cardiovasc. Pharmacol. 2000, 36, 493–497.
- Li, J.; Qi, D.; Cheng, H.; Hu, X.; Miller, E.; Wu, X.; Russell, K.S.; Mikush, N.; Zhang, J.; Xiao, L.; et al. Urocortin 2 autocrine/paracrine and pharmacologic effects to activate AMP-activated protein kinase in the heart. Proc. Natl. Acad. Sci. USA 2013, 110, 16133–16138.
- Chen, A.; Blount, A.; Vaughan, J.; Brar, B.; Vale, W. Urocortin II Gene Is Highly Expressed in Mouse Skin and Skeletal Muscle Tissues: Localization, Basal Expression in Corticotropin-Releasing Factor Receptor (CRFR) 1- and CRFR2-Null Mice, and Regulation by Glucocorticoids. Endocrinology 2004, 145, 2445–2457.
- Takahashi, K.; Totsune, K.; Murakami, O.; Saruta, M.; Nakabayashi, M.; Suzuki, T.; Sasano, H.; Shibahara, S. Expression of Urocortin III/Stresscopin in Human Heart and Kidney. J. Clin. Endocrinol. Metab. 2004, 89, 1897–1903.
- Takahashi, K. Distribution of Urocortins and Corticotropin-Releasing Factor Receptors in the Cardiovascular System. Int. J. Endocrinol. 2012, 2012, 395284.
- Tsuda, T.; Takefuji, M.; Wettschureck, N.; Kotani, K.; Morimoto, R.; Okumura, T.; Kaur, H.; Eguchi, S.; Sakaguchi, T.; Ishihama, S.; et al. Corticotropin releasing hormone receptor 2 exacerbates chronic cardiac dysfunction. J. Exp. Med. 2017, 214, 1877–1888.
- Yarur, H.E.; Andrés, M.E.; Gysling, K. Type 2β Corticotrophin Releasing Factor Receptor Forms a Heteromeric Complex With Dopamine D1 Receptor in Living Cells. Front. Pharmacol. 2020, 10, 1501.
- Kishimoto, T.; Pearse, R.; Lin, C.R.; Rosenfeld, M.G. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc. Natl. Acad. Sci. USA 1995, 92, 1108–1112.
- Pilbrow, A.P.; Lewis, K.A.; Perrin, M.H.; Sweet, W.E.; Moravec, C.S.; Tang, W.H.W.; Huising, M.O.; Troughton, R.; Cameron, V. Cardiac CRFR1 Expression Is Elevated in Human Heart Failure and Modulated by Genetic Variation and Alternative Splicing. Endocrinology 2016, 157, 4865–4874.
- Squillacioti, C.; Pelagalli, A.; Liguori, G.; Mirabella, N. Urocortins in the mammalian endocrine system. Acta Veter.-Scand. 2019, 61, 46.
- Brar, B.K.; Stephanou, A.; Knight, R.; Latchman, D.S. Activation of Protein Kinase B/Akt by Urocortin is Essential for its Ability to Protect Cardiac Cells Against Hypoxia/Reoxygenation-induced Cell Death. J. Mol. Cell. Cardiol. 2002, 34, 483–492.
- Calderón-Sánchez, E.M.; Delgado, C.; Ruiz-Hurtado, G.; Domínguez-Rodríguez, A.; Cachofeiro, V.; Rodríguez-Moyano, M.; Gomez, A.M.; Ordonez, A.; Smani, T.; Hajami, T.S. Urocortin induces positive inotropic effect in rat heart. Cardiovasc. Res. 2009, 83, 717–725.
- Walther, S.; Pluteanu, F.; Renz, S.; Nikonova, Y.; Maxwell, J.T.; Yang, L.-Z.; Schmidt, K.; Edwards, J.N.; Wakula, P.; Groschner, K.; et al. Urocortin 2 stimulates nitric oxide production in ventricular myocytes via Akt- and PKA-mediated phosphorylation of eNOS at serine 1177. Am. J. Physiol. Circ. Physiol. 2014, 307, H689–H700.
- Popov, S.V.; Prokudina, E.S.; Mukhomedzyanov, A.V.; Naryzhnaya, N.V.; Ma, H.; Zurmanova, J.M.; van der Ven, P.F.M.; Maslov, L.N. Cardioprotective and Vasoprotective Effects of Corticotropin-Releasing Hormone and Urocortins: Receptors and Signaling. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 575–584.
- Okosi, A.; Brar, B.; Chan, M.; D’Souza, L.; Smith, E.; Stephanou, A.; Latchman, D.; Chowdrey, H.; Knight, R. Expression and protective effects of urocortin in cardiac myocytes. Neuropeptides 1998, 32, 167–171.
- Scarabelli, T.M.; Pasini, E.; Stephanou, A.; Comini, L.; Curello, S.; Raddino, R.; Ferrari, R.; Knight, R.; Latchman, D.S. Urocortin promotes hemodynamic and bioenergetic recovery and improves cell survival in the isolated rat heart exposed to ischemia/reperfusion. J. Am. Coll. Cardiol. 2002, 40, 155–161.
- Janjua, S.; Lawrence, K.M.; Ng, L.L.; Latchman, D.S. The cardioprotective agent urocortin induces expression of CT-1. Cardiovasc. Toxicol. 2003, 3, 255–262.
- Lawrence, K.M.; Kabir, A.M.N.; Bellahcene, M.; Davidson, S.; Mesquita, R.S.; Cao, X.; McCormick, J.; Carroll, C.; Chanalaris, A.; Townsend, P.A.; et al. Cardioprotection mediated by urocortin is dependent upon PKCε activation. FASEB J. 2005, 19, 1–18.
- Domínguez-Rodríguez, A.; González, I.M.; Avila-Medina, J.; Pedro, E.S.D.R.-D.; Calderón-Sánchez, E.; Díaz, I.; Hmadcha, A.; Castellano, A.; Rosado, J.; Benitah, J.-P.; et al. Urocortin-2 Prevents Dysregulation of Ca2+ Homeostasis and Improves Early Cardiac Remodeling After Ischemia and Reperfusion. Front. Physiol. 2018, 9, 813.
- Valentim, L.; Laurence, K.M.; Townsend, P.; Carroll, C.; Soond, S.; Scarabelli, T.M.; Knight, R.A.; Latchman, D.S.; Stephanou, A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J. Mol. Cell. Cardiol. 2006, 40, 846–852.
- Calderón-Sánchez, E.M.; Díaz, I.; Ordoñez, A.; Smani, T. Urocortin-1 Mediated Cardioprotection Involves XIAP and CD40-Ligand Recovery: Role of EPAC2 and ERK1/2. PLoS ONE 2016, 11, e0147375.
- Gao, X.-F.; Zhou, Y.; Wang, D.-Y.; Lew, K.-S.; Richards, A.M.; Wang, P. Urocortin-2 suppression of p38-MAPK signaling as an additional mechanism for ischemic cardioprotection. Mol. Cell. Biochem. 2014, 398, 135–146.
- Lawrence, K.; Chanalaris, A.; Scarabelli, T.; Hubank, M.; Pasini, E.; Townsend, P.; Comini, L.; Ferrari, R.; Tinker, A.; Stephanou, A.; et al. K ATP Channel Gene Expression Is Induced by Urocortin and Mediates Its Cardioprotective Effect. Circulation 2002, 106, 1556–1562.
- Cong, B.; Wang, L.; Zhu, X.; Li, X.; Liu, B.; Ni, X. SGK1 Is Involved in Cardioprotection of Urocortin-1 Against Hypoxia/Reoxygenation in Cardiomyocytes. Can. J. Cardiol. 2014, 30, 687–695.
- Zhou, Y.; Chen, Q.; Lew, K.S.; Richards, A.M.; Wang, P. Discovery of Potential Therapeutic miRNA Targets in Cardiac Ischemia–Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 296–309.
- Díaz, I.; Calderón-Sánchez, E.M.; Del Toro, R.; Avila-Medina, J.; Pedro, E.S.D.R.-D.; Domínguez-Rodríguez, A.; Rosado, J.A.; Hmadcha, A.; Ordoñez, A.; Smani, T. miR-125a, miR-139 and miR-324 contribute to Urocortin protection against myocardial ischemia-reperfusion injury. Sci. Rep. 2017, 7, 8898.
- Smani, T.; González, I.M.; Galeano-Otero, I.; Gallardo-Castillo, I.; Rosado, J.A.; Ordoñez, A.; Hmadcha, A. Non-coding RNAs and Ischemic Cardiovascular Diseases. Adv. Exp. Med. Biol. 2020, 1229, 259–271.
More
