Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Vanessa Freitas.
Extracellular vesicles are lipid bilayer-enclosed particles released from all types of cells and found in biological fluids, which transport variable content and have crucial functions in cell–cell communication. The role of extracellular vesicles in cancer is a current hot topic, and no bibliometric study has ever analyzed research production regarding their role in breast cancer and indicated the trends in the field. In this way, wthe study aimed to investigate the trends in breast cancer management involved with extracellular vesicle research.
- breast cancer
- metastasis
- exosomes
- extracellular vesicles
- bibliometrics
1. Bibliometrics Findinds in Extracellular Vesicle research and Breast cancer management
Our team has conducted a investigation about the publications involving breast cancer and extracellular vesicles. The search resulted in 1151 documents, in which the first article involving exosomes and breast cancer was published in 1998; however, the first use of the term “exosomes” was in 1970[1]. These documents accounted for 785 experimental and clinical articles and 295 reviews; the other 71 included different document types, such as entire books or individual chapters, conference papers, and editorial notes. The documents were published in 451 different sources, cited 41,363 times in total, and written by 5599 authors from 57 countries.
An increasing number of publications can be observed (Figure 1), wherein one article was published in 1998—the first one—and the curve reached its peak of 318 articles in 2020, with 276 more published articles until the moment of the data update (October 2021). The mean of the total citations per year normalized by the citable years is shown in. It evidences some of the peaks of the citations that are correlated with the important articles published on the theme, which had a great impact and directed the research in the field, also making it possible to create a historiographic map to retrieve these impactful articles (Figure 2).
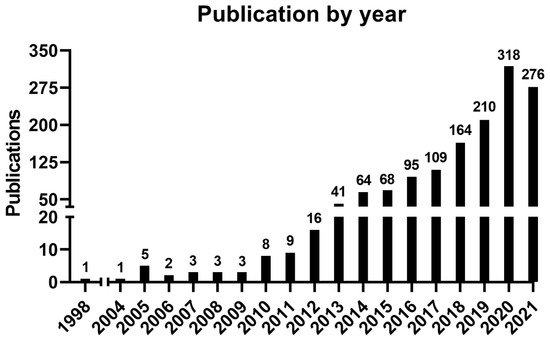
Figure 1. Number of publications by year.
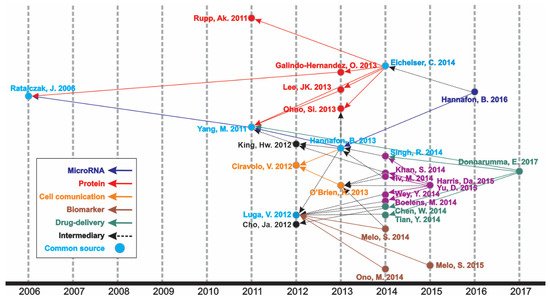
Figure 2. Historical direct citation network.
In the historiographic map, different groups of articles are observed indicating a scientific path throughout the years of publications on the theme, as indicated in Figure 2. Thus, the major path, meaning from the last impactful publication on the theme (in 2017) until the first one (in 2006), is constituted by five minor paths (MicroRNA, Protein, Cell communication, Biomarker, and Drug delivery), with some articles contributing to more than one minor path or presenting content of transition, respectively nominated as “Common source” and “Intermediary” paths.
The most relevant source regarding the number of articles published was the International Journal of Molecular Sciences (38 documents, IF: 4.556), followed by Oncotarget (37 documents, IF: 5.168 in 2016) and Scientific Reports (32 documents, IF: 3.998). Taken together, these three journals represented 9.2% of all the publications on the theme.
However, the number of publications was not correlated with the citation number. Thus, four journals (Oncotarget, Plos One, Scientific Reports, and Cancer Research) can be found in different positions in both tables, indicating that a higher citation number is not correlated with the higher impact of publications and citations of a journal. The top three most cited sources were “Cancer Research” (2116 citations, IF: 9.727), “Nature” (1491 citations, IF: 42.779), and “Oncotarget” (1447 citations, IF: 5.168 *). Coincidently, 8 out of 10 journals listed were also among the 50 journals that contributed the most, accounting for the top 100 most cited articles in extracellular vesicles and cancer[2]. “Cancer Research”, “Plos One”, “Nature”, and “Cell and Nature Biology” were the top 10 in both ranks. The top 10 journals comprise 27.5% of all citations.
The most cited article was “Glypican-1 identifies cancer exosomes and detects early pancreatic cancer”, written by Sonia A. Melo [3], published in “Nature”, and cited 1561 times. In this paper, a cell surface proteoglycan (glypican-1) was identified as being specifically enriched on cancer cell-derived exosomes, serving as a potential non-invasive diagnostic and screening tool to detect the early stages of pancreatic cancer. Moreover, it is directly involved in the historical direct citation network (Figure 2), contributing to guiding other studies throughout the research on this theme.
The institution presenting the highest production regarding the number of publications was “Cornell University”, USA, accounting for 36 publications, followed by “The University of California”, USA, with 35 publications and “Shahid Beheshti University of Medical Sciences”, Iran, with 33 publications. The top 10 most productive institutions are from the USA, Iran, and China, representing a closely distributed production. Concerning the most productive country, the USA ranked as the first, in which all its institutions published 355 articles, followed by China, which published 311 articles, and Italy, which published 83 articles (Figure 3).
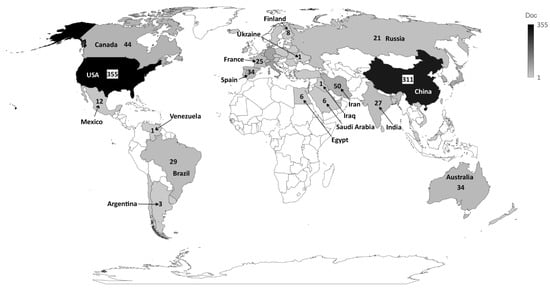
Figure 3. Countries that published most on the theme.
A total of 2146 author keywords were retrieved, and 45 met the threshold of a minimum number of occurrences at 10 times cited. These keywords were grouped into six clusters (Figure 4), presenting a different average of citation (Figure 5) and different years of appearance in the articles on the theme (Figure 6). The keywords with at least 10 occurrences, in the top 100 most cited articles in cancer and extracellular vesicles[2], roughly coincided with those we found. For instance, “microRNA”, “diagnostic biomarker”/“diagnosis” and “biomarker”, “proteomic analysis”/“proteomics”, “angiogenesis”, and “metastasis”. Interestingly, “chemotherapy” and “drug delivery”, included in our keyword network with approximately 40 citations, were not found in the mentioned previous study. This suggests that the research in breast cancer and extracellular vesicles may have been following an additional path for the last 4 years.
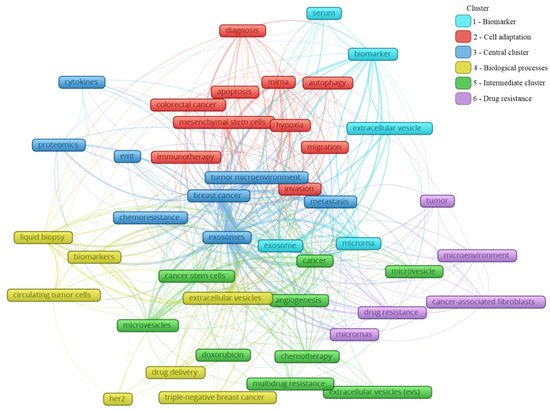
Figure 4. Main keyword clusters related to extracellular vesicles and breast cancer.
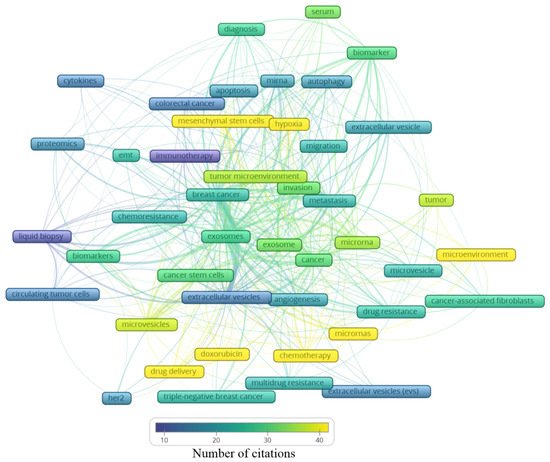
Figure 5. Average number of citations of the main keywords related to extracellular vesicles and breast cancer.
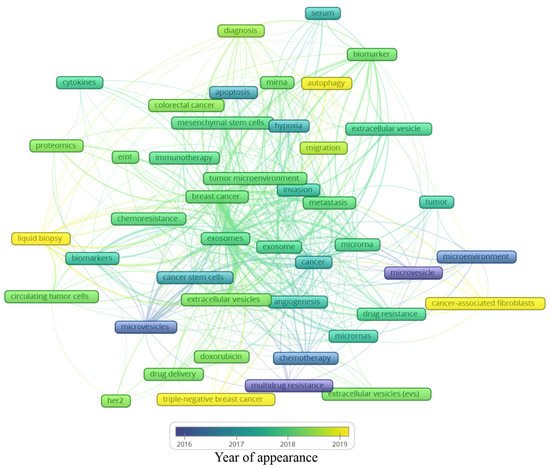
Figure 6. Average year of appearance of the main keywords related to extracellular vesicles and breast cancer.
In Figure 4, the clusters are grouped by colors. From the cluster “1—Biomarker”, the keywords point to the microRNA of extracellular vesicles being used as biomarkers, mainly from the serum. The cluster “2—Cell adaptation” presents the process of autophagy and cell migration as being associated with a hypoxic microenvironment, probably having an influence on mesenchymal stem cells in colorectal cancer progression, and that could be treated with immunotherapy; additionally, miRNA is pointed as a strategy to be used in the diagnosis of colorectal cancer, as well as breast cancer. In cluster “6—Drug resistance”, an association between cancer-associated fibroblasts (CAFs) and microRNAs in the drug resistance process of the tumor microenvironment can be observed. The cluster “4—Biological processes” shows that drug delivery can be a strategy in the treatment of HER-2 and triple-negative breast cancer, and to improve the diagnosis accuracy, a liquid biopsy analyzing circulating tumor cells or using extracellular vesicles as biomarkers can constitute the next scientific direction on the theme. The cluster “5—Intermediate cluster” is an intermediate between the “6—Drug resistance” and the “4—Biological processes” clusters, in which the association between the tumor microenvironment and biological processes, such as angiogenesis, being mediated by microvesicles and not exclusively by exosomes, is displayed. Moreover, microvesicles and exosomes seem to influence other scenarios in cancer treatment, for example, the multidrug resistance to chemotherapeutics classically used in breast cancer, such as doxorubicin. In the final cluster, “3—Central cluster”, the main keywords are grouped, which are linked with all the clusters.
Based on these findings, it is reasonable to assume that exosomes might play a great role in the breast cancer microenvironment and metastasis development. Likewise, the chemoresistance process can be facilitated by these particles, representing a negative impact on the clinical setting. Proteomics was the methodology mostly used; it is associated with cytokine analysis, which is also linked with epithelial–mesenchymal transition, known to be essential to tumor progression and metastasis development.
Analyzing the clusters by average citation (Figure 5), the keywords most cited are related to drug delivery, in this case, doxorubicin, as well the influence of microRNAs on the tumor microenvironment, participating in the angiogenic process or even drug resistance. Moreover, hypoxia and mesenchymal stem cells showed an association with the tumor microenvironment, the invasion process, and metastasis. Exosomes and microvesicles were not often cited but are both in the center of the map as well as in each cluster, indicating their relevance in different scenarios regarding breast cancer.
Analyzing the year of appearance (Figure 6), the classic keywords on the theme, presenting themselves as the oldest, are “microvesicles”, “multidrug resistance”, and “microenvironment”. As the research was directed toward biological processes, other keywords were evidenced, such as “apoptosis”, “hypoxia”, and “angiogenesis”. Following the advances in the field, the next steps in treatment approaches were observed, for instance, “drug delivery”, “doxorubicin”, and “chemoresistance”. In recent years, a mixture of themes is present, with current hotspots “autophagy”, “liquid biopsy”, “cancer-associated fibroblasts”, and “triple-negative breast cancer”. The keywords and their different interactions in each map cannot be analyzed alone; it is important to consider all the links around each hotspot. In this manner, a general idea can be constructed around the newest keywords (Figure 6).
The keyword “autophagy” is linked to “extracellular vesicle”, “migration”, “tumor microenvironment”, and “miRNA”. Autophagy is a conserved catabolic process, in which cells degrade defective cytoplasmic molecules and organelles through lysosomal activity. This process is triggered by the communication of the cell with its microenvironment, being a response to external stress factors and an attempt to reach homeostasis[3]. Its vesicular machinery is involved in the exocytosis of extracellular vesicles through the fusion of the membrane of late endosomes with the cell membrane[4], establishing a route of communication between autophagic cancer cells and its milieu[3]. Autophagy can have a dual effect on cancer cells, suppressing (e.g., inhibit tumor growth) or promoting (e.g., maintenance of stem properties on cancer stem cells) tumorigenesis[5]. Hamurcu et al. (2017) observed that the triple-negative breast cancer cell line MDA-MB-231 has a higher rate of autophagy and expression of autophagy-related proteins LC3 and Beclin. Moreover, the downregulation of those proteins led to the suppression of autophagy, migration, proliferation, and colony formation potential and increased apoptosis [6] Regarding the relation between “autophagy” and “miRNA”, the inhibitory potential of miRNA has been studied in this process. For example, the delivery of miRNA-376b through nanoparticles on breast cancer cells led to a reduction in tumor mass in a xenograft mice model and presented an even stronger effect when used in combination with cisplatin[7].
The keyword “cancer-associated fibroblasts” is linked to “metastasis”, “microvesicles”, “cancer”, and “microRNAs”. The tumor microenvironment is rich in stromal cells, such as fibroblasts, and those cells can be re-programmed due to the endocytosis of tumor-derived extracellular vesicles containing TGF-B1, TGF-B2, IL-6, MMP-2, and MMP-9 [8]. There is increasing evidence that these cancer-associated fibroblasts (CAFs) contribute to tumor progression and metastasis, placing it in the spotlight for novel anti-tumoral therapies[9]. CAF-derived EVs have different contents when compared with normal fibroblasts, for example, presenting the downregulation of miR-1-3p (a miRNA can inhibit the cell viability, invasion, migration, and epithelial–mesenchymal transition of breast cancer cells) when compared with normal fibroblasts. Then, Tao et al. (2020) transfected a miR-1-3p mimic into CAF-derived EVs, showing the potential of those cells in the delivery of the antineoplastic miRNA[10]. On the other hand, CAFs may modulate healthy and neoplastic cells through EVs, such as exosomes and microvesicles. For example, CD81+ exosomes are shed by CAFs and can be internalized by breast cancer cells, altering Wnt pathway molecules, which may contribute to an increase in cell protrusions and motility, favoring the metastasis cascade[11]. Moreover, exosomes seem to be involved in epithelial–mesenchymal transition, a key process in metastasis[12]. Sansone et al. (2017) showed the contribution of CAF-derived microvesicles in the acquisition of stemness properties of luminal breast cancer cells through the transference of miR-221, promoting resistance to hormonal therapies[13].
The keyword “triple-negative breast cancer” is linked to “extracellular vesicles”, “angiogenesis”, “drug delivery”, and “doxorubicin”. The classification of breast cancer can be based on the expression of the receptors for estrogen, progesterone, and HER2. The triple-negative (TN) subtype has a low expression of those hormone receptors and does not respond to hormone-based therapies, presenting a highly invasive nature, commonly evolving to metastatic disease[14]. Our group has demonstrated that the human TN cell line MDA-MB-231-derived EVs are more internalized by fibroblasts, and vice versa, than EVs derived from MCF-7, a luminal human cancer cell line[15]. TN breast cancer EVs can also interact with other cell types, such as endothelial cells, contributing to tumor progression[16]. In another study, it was observed that the association of breast cancer EVs and annexin 2A plays an important role in angiogenesis and the formation of a pre-metastatic niche through the activation of macrophages[17]. In a later publication of the same group, they correlated the previous association with the high incidence and aggressiveness of TN breast cancer in African American women[18]. EVs also show a promising antitumoral effect if employed as a drug delivery system. Gong et al. (2019) developed a delivery system using EVs secreted by human monocyte cells rich in a modified version of disintegrin and metalloproteinase-15 (A15), which binds to the integrin αvβ3 present in TN breast cancer cells. They tested the co-delivery of the chemotherapeutic agent doxorubicin and microRNA-159 and observed synergic anticancer effects both in in vitro and in vivo models of TN breast cancer[19]. In a similar study, Haney et al. (2019) demonstrated that monocyte EVs loaded with doxorubicin and paclitaxel induced the accumulation of those drugs inside of TN breast cancer cells in vitro and in vivo, increasing cytotoxicity and suppressing tumor formation, respectively [20].
Finally, the keyword “liquid biopsy” seems to be the most relevant among the newest keywords since it has many links, which are “breast cancer”, “biomarkers”, “diagnosis”, “extracellular vesicles”, “exosomes”, and “circulating tumor cells”. A liquid biopsy consists of a less invasive strategy for the detection of cancer relying on the molecular identification of the contents released from tumor cells, such as DNA, miRNA, and other biomarkers present in the blood, serum, and plasma [21]. Those molecules can be found free in those fluids or in circulating cancer cells or EVs. It has been demonstrated by several studies that EVs shed by breast cancer cells may contain stable miRNAs, such as miR-21 and miR-1246 in exosomes, constituting a potential tool in the future of breast cancer detection and diagnosis. However, there is still no consensus in the scientific community about which specific miRNAs could be used for this purpose. Hence, a liquid biopsy could allow the detection of cancer, prognosis, and could even guide therapy taking into account the molecular pattern of the tumor content present in the plasma of patients[22]. The growing research on non-coding RNA carried by extracellular vesicles goes beyond miRNAs, including ncRNAs, tRNA and tRNA fragments, Y RNA, piRNA, rRNA, and lncRNA, and requires a separate bibliometric study.
Additional information
Additional information can be fiound in https://doi.org/10.3390/curroncol28060382.
References
- Chul Won Yun; Sang Hun Lee; The Roles of Autophagy in Cancer. International Journal of Molecular Sciences 2018, 19, 3466, 10.3390/ijms19113466.
- Zuhal Hamurcu; Nesrin Delibaşı; Seda Geçene; Elif Funda Şener; Hamiyet Dönmez-Altuntaş; Yusuf Özkul; Halit Canatan; Bulent Ozpolat; Targeting LC3 and Beclin-1 autophagy genes suppresses proliferation, survival, migration and invasion by inhibition of Cyclin-D1 and uPAR/Integrin β1/ Src signaling in triple negative breast cancer cells. Journal of Cancer Research and Clinical Oncology 2017, 144, 415-430, 10.1007/s00432-017-2557-5.
- Ozlem Unal; Yunus Akkoc; Muhammed Kocak; Esra Nalbat; Asiye Isin Dogan-Ekici; Havva Yagci Acar; Devrim Gozuacik; Treatment of breast cancer with autophagy inhibitory microRNAs carried by AGO2-conjugated nanoparticles. Journal of Nanobiotechnology 2020, 18, 1-18, 10.1186/s12951-020-00615-4.
- Petr Heneberg; Paracrine tumor signaling induces transdifferentiation of surrounding fibroblasts. Critical Reviews in Oncology/Hematology 2015, 97, 303-311, 10.1016/j.critrevonc.2015.09.008.
- Erik Sahai; Igor Astsaturov; Edna Cukierman; David G. DeNardo; Mikala Egeblad; Ronald Evans; Douglas Fearon; Florian R. Greten; Sunil R. Hingorani; Tony Hunter; et al.Richard O. HynesRakesh K. JainTobias JanowitzClaus JorgensenAlec C. KimmelmanMikhail G. KoloninRobert G. MakiR. Scott PowersEllen PuréDaniel C. RamirezRuth Scherz-ShouvalMara H. ShermanSheila StewartThea D. TlstyDavid A. TuvesonFiona M. WattValerie WeaverAshani T. WeeraratnaZena Werb A framework for advancing our understanding of cancer-associated fibroblasts. Nature Reviews Cancer 2020, 20, 174-186, 10.1038/s41568-019-0238-1.
- Shuang Tao; Hong Li; Xiuzhen Ma; Yunfei Ma; Jiale He; Yali Gao; Jinping Li; Elevating microRNA-1-3p shuttled by cancer-associated fibroblasts-derived extracellular vesicles suppresses breast cancer progression and metastasis by inhibiting GLIS1. Cancer Gene Therapy 2020, 28, 634-648, 10.1038/s41417-020-00244-x.
- Valbona Luga; Jeffrey L. Wrana; Tumor–Stroma Interaction: Revealing Fibroblast-Secreted Exosomes as Potent Regulators of Wnt-Planar Cell Polarity Signaling in Cancer Metastasis. Cancer Research 2013, 73, 6843-6847, 10.1158/0008-5472.can-13-1791.
- David Greening; Shashi K. Gopal; Rommel Mathias; Lin Liu; Jingyi Sheng; Hong-Jian Zhu; Richard J. Simpson; Emerging roles of exosomes during epithelial–mesenchymal transition and cancer progression. Seminars in Cell & Developmental Biology 2015, 40, 60-71, 10.1016/j.semcdb.2015.02.008.
- Pasquale Sansone; Marjan Berishaj; Vinagolu K. Rajasekhar; Claudio Ceccarelli; Qing Chang; Antonio Strillacci; Claudia Savini; Lauren Shapiro; Robert L. Bowman; Chiara Mastroleo; et al.Sabrina De CarolisLaura DalyAlberto Benito-MartinFabiana PernaNicola FabbriJohn HealeyEnzo SpisniMonica CriccaDavid LydenMassimiliano BonaféJacqueline Bromberg Evolution of Cancer Stem-like Cells in Endocrine-Resistant Metastatic Breast Cancers Is Mediated by Stromal Microvesicles.. Cancer Research 2017, 77, 1927-1941, 10.1158/0008-5472.CAN-16-2129.
- Mauricio A. Medina; Goldie Oza; Ashutosh Sharma; L.G. Arriaga; José Manuel Hernández Hernández; Vincent M. Rotello; Jose Tapia Ramirez; Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. International Journal of Environmental Research and Public Health 2020, 17, 2078, 10.3390/ijerph17062078.
- Thaiomara A. Silva; Basílio Smuczek; Iuri C. Valadão; Luciana M. Dzik; Rebeca Iglesia; Mário C. Cruz; André Zelanis; Adriane S. De Siqueira; Solange M T Serrano; Gary S. Goldberg; et al.Ruy JaegerVanessa M. Freitas AHNAK enables mammary carcinoma cells to produce extracellular vesicles that increase neighboring fibroblast cell motility. Oncotarget 2016, 7, 49998-50016, 10.18632/oncotarget.10307.
- Shinsuke Kikuchi; Yusuke Yoshioka; Marta Prieto-Vila; Takahiro Ochiya; Involvement of Extracellular Vesicles in Vascular-Related Functions in Cancer Progression and Metastasis. International Journal of Molecular Sciences 2019, 20, 2584, 10.3390/ijms20102584.
- Sayantan Maji; Pankaj Chaudhary; Irina Akopova; Phung M. Nguyen; Richard J. Hare; Ignacy Gryczynski; Jamboor Vishwanatha; Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Molecular Cancer Research 2016, 15, 93-105, 10.1158/1541-7786.mcr-16-0163.
- Pankaj Chaudhary; Lee Gibbs; Sayantan Maji; Cheryl M. Lewis; Sumihiro Suzuki; Jamboor K. Vishwanatha; Serum exosomal-annexin A2 is associated with African-American triple-negative breast cancer and promotes angiogenesis. Breast Cancer Research 2020, 22, 1-15, 10.1186/s13058-020-1251-8.
- Chunai Gong; Jing Tian; Zhuo Wang; Yuan Gao; Xin Wu; Xueying Ding; Lei Qiang; Guorui Li; Zhimin Han; Yongfang Yuan; et al.Shen Gao Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. Journal of Nanobiotechnology 2019, 17, 1-18, 10.1186/s12951-019-0526-7.
- Matthew J. Haney; Yuling Zhao; Yeon S. Jin; Samuel M. Li; Juli R. Bago; Natalia L. Klyachko; Alexander V. Kabanov; Elena V. Batrakova; Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy. Journal of Neuroimmune Pharmacology 2019, 15, 487-500, 10.1007/s11481-019-09884-9.
- Geoffroy Poulet; Joséphine Massias; Valerie Taly; Liquid Biopsy: General Concepts. Acta Cytologica 2019, 63, 449-455, 10.1159/000499337.
- Patricia Midori Murobushi Ozawa; Tayana Schultz Jucoski; Evelyn Vieira; Tamyres Mingorance Carvalho; Danielle Malheiros; Enilze Maria De Souza Fonseca Ribeiro; Liquid biopsy for breast cancer using extracellular vesicles and cell-free microRNAs as biomarkers. Translational Research 2020, 223, 40-60, 10.1016/j.trsl.2020.04.002.
- Agata Abramowicz; Michael D Story; The Long and Short of It: The Emerging Roles of Non-Coding RNA in Small Extracellular Vesicles. Cancers 2020, 12, 1445, 10.3390/cancers12061445.
- A. S. Fox; S. B. Yoon; DNA-Induced Transformation in Drosophila: Locus-Specificity and the Establishment of Transformed Stocks. Proceedings of the National Academy of Sciences 1970, 67, 1608-1615, 10.1073/pnas.67.3.1608.
- Shuzhen Shi; Ya Gao; Ming Liu; Youxiang Bu; Jiarui Wu; Jinhui Tian; Junhua Zhang; Top 100 most-cited articles on exosomes in the field of cancer: a bibliometric analysis and evidence mapping. Clinical and Experimental Medicine 2020, 21, 181-194, 10.1007/s10238-020-00624-5.
- Jing Xu; Robert Camfield; Sharon M. Gorski; The interplay between exosomes and autophagy – partners in crime. Journal of Cell Science 2018, 131, jcs215210, 10.1242/jcs.215210.
More
