Astringency has Latin origin from the word
ad stringere, which means “to bind”. It is commonly referred to as the dry mouthfeel, although it is a very complex sensation with various definitions proposed over time. It is as a complex phenomenon that provokes a range of sensations, triggered by different types of substances, and explained by diverse mechanisms. The American Society for Testing and Materials (ASTM) defines it as "the complex of sensations due to shrinking, drawing or puckering of the epithelium as a result of exposure to substances such as alums or tannins". The astringency perception arises from the interaction of astringents with the oral cavity, e.g., tissues, cell membrane proteins, epithelial cells, mechano, and chemo-receptors. Consequently, many mechanisms, beyond simple lubrication, drive this mouthfeel.
which means “to bind”. It is commonly referred to as the dry mouthfeel, although it is a very complex sensation with various definitions proposed over time. It is as a complex phenomenon that provokes a range of sensations, triggered by different types of substances, and explained by diverse mechanisms. The American Society for Testing and Materials (ASTM) defines it as “the complex of sensations due to shrinking, drawing or puckering of the epithelium as a result of exposure to substances such as alums or tannins”. The astringency perception arises from the interaction of astringents with the oral cavity, e.g., tissues, cell membrane proteins, epithelial cells, mechano, and chemo-receptors. Consequently, many mechanisms, beyond simple lubrication, drive this mouthfeel.
- sensorial perception
- astringency
- oral mechanisms
- lubrication
- salivary proteins
- protein-food interactions
1. Introduction
1. Abstract
Understanding consumers’ food choices and the psychological processes involved in their preferences is crucial to promote more mindful eating regulation and guide food design. Fortifying foods minimizing the oral dryness, rough, and puckering associated with many functional ingredients has been attracting interest in understanding oral astringency over the years. A variety of studies have explored the sensorial mechanisms and the food properties determining astringency perception. Here we provide a deeper understanding of astringency, a general view of the oral mechanisms involved, and the exciting variety of the latest methods used to direct and indirectly quantify and simulate the astringency perception and the specific mechanisms involved.
2. Introduction
1.1. Astringency
2.1. Astringency
The human being consciously interacts with the surrounding environment through its five senses, which determines the sensory perception. Perception is defined in the Oxford dictionary as "the awareness through the senses interpreted in the light of experience". In other words, sensorial perception is the consciousness arising through a single sense or a combination of multiple senses with personal factors. The oral perception of food is the result of food features interacting in the mouth and immediately interpreted by the brain
The human being consciously interacts with the surrounding environment through its five senses, which determines the sensory perception. Perception is defined in the Oxford dictionary as “the awareness through the senses interpreted in the light of experience”. In other words, sensorial perception is the consciousness arising through a single sense or a combination of multiple senses with personal factors. The oral perception of food is the result of food features interacting in the mouth and immediately interpreted by the brain
[1]
. The sensory responses to the taste, aroma, color, and texture of foods further determine the food preferences and the eating habits of the consumers.
Given the recent trend to fortify consumables with functional ingredients and simultaneously minimize their sometimes undesired mouthfeel, the mechanisms of oral development of astringency have attracted more interest
[2]
. Astringency is commonly referred to as the dry mouthfeel, although it is a very complex sensation with various definitions proposed over time.
Astringency has Latin origin from the word
ad stringere, which means "to bind". It was once considered a basic taste modality in ancient Indian culture. However, since then, it was understood as a tactile sensation due to the mechanical effect of the decreased salivary lubrication
, which means “to bind”. It was once considered a basic taste modality in ancient Indian culture. However, since then, it was understood as a tactile sensation due to the mechanical effect of the decreased salivary lubrication
[3]
. In the earlier years, Bate-Smith et al.
[4]
suggested it was a feeling, not a taste. They were opposed to the explanation of astringency as being an additional taste to the five accepted gustatory sensations (i.e., sweet, sour, salty, bitter, and umami). They reported it as an event induced by tannin interaction and precipitation of salivary proline-rich proteins (PRPs) in the oral cavity. Indeed, Joslyn and Goldstein
[5], who advocated this theory at the time, promoted the tactile theory of astringency. They stated the "precipitation of tissue proteins is accompanied by shrinkage of tissue due to water loss and a decrease in the permeability of this tissue to water and solutes". Furthermore, Lawless and Corrigan
, who advocated this theory at the time, promoted the tactile theory of astringency. They stated the “precipitation of tissue proteins is accompanied by shrinkage of tissue due to water loss and a decrease in the permeability of this tissue to water and solutes”. Furthermore, Lawless and Corrigan
[6]
also defined astringency as a more physical event, referring it to the tightening and drawing sensations felt in the buccal musculature and to the sensations of drying and roughness when there are contact and movement in the mouth. This general concept view has been enduring. However, it became unclear whether astringent compounds trigger mechanosensation, chemosensation, or a combination of both. Later, Peleg H. et al.
[7] reported astringency as a complex phenomenon that provokes a range of sensations, triggered by different types of substances, and explained by diverse mechanisms. The American Society for Testing and Materials (ASTM) defines it as "the complex of sensations due to shrinking, drawing or puckering of the epithelium as a result of exposure to substances such as alums or tannins"
reported astringency as a complex phenomenon that provokes a range of sensations, triggered by different types of substances, and explained by diverse mechanisms. The American Society for Testing and Materials (ASTM) defines it as “the complex of sensations due to shrinking, drawing or puckering of the epithelium as a result of exposure to substances such as alums or tannins”
[8]
.
1.2. General Mechanisms of Astringency
2.2. General Mechanisms of Astringency
The astringency perception arises from the interaction of astringents with the oral cavity, e.g., tissues, cell membrane proteins, epithelial cells, mechano, and chemo-receptors. Consequently, many mechanisms, beyond simple lubrication, drive this mouthfeel
[9]
.
The oral cavity is coated and lubricated with a salivary film, which is significantly impaired by the consumption of food and beverages
[10]
. The film is composed of two distinct layers: a dynamic and easily removable part and a pellicle layer covalently attached to the epithelial cells
[11]
. The latter one has a thickness around 2 to 100 nm, and its disruption exposes cell surface and receptors, leading to a high surface hydrophobicity (Water Contact Angle 71°), and friction increase
[12]
. Gibbins and Carpenter
[13]
have indicated that several events involving the astringent compounds and the saliva cause oral surface properties alterations, changes in the rheological, and lubricating properties, and possibly the further activation of cell receptors. According to these same authors, hypothetical and underlying mechanisms related to astringency are protein precipitation, breakage of the salivary pellicle, decrease in salivary lubrication, mechanical perception sensed by receptors (mechano and chemoreceptors) and shrinkage of tissues, i.e., mainly changes in oral epithelium. A schematic representation of this mechanism is depicted in
. Furthermore, Ployon et al. used a model of the oral mucosa, demonstrating that the aggregation of the mucosal pellicle leads to an increase of the friction forces at the mucosa surface. The authors also showed the protective role of PRP regarding the aggregation of the mucosal pellicle
[14]
. Despite the proposed general events, a more in-depth understanding of the continuity and properties of the salivary pellicle and its disruption by the astringents is still needed in order to validate this holistic point of view
. The most studied mechanism of astringency is the interaction between salivary proteins (e.g., PRPs) and the food compounds. Several works have shown good correlations between the perceived astringency
[15]
and the interaction of the astringent with proteins (from saliva, oral mucosa, or cells), e.g., in terms of polyphenol precipitation, effects of salts, polymer, and ionic strength
. The following sub-sections explore reported studies on the interactions with polyphenols.
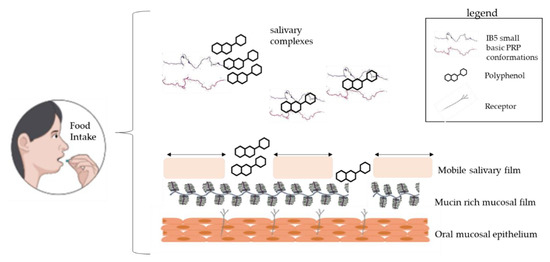
Figure 1. Possible mechanisms of astringency occurring in the oral cavity: aggregation of salivary proteins creating grittiness, salivary film disruption, reduced salivary lubrication, and possible exposure of cell receptors. Schematic based on
[13]
.
1.3. Compounds Causing Astringency
2.3. Compounds Causing Astringency
Numerous and powerful health benefits of some astringent, such as polyphenolic compounds, were reported. However, the ingestion of polyphenol-rich foods and beverages is frequently associated with a tactile dryness and roughness, and constriction perceived throughout the oral cavity. Moreover, tannins can have antinutritional effects, generally attributed to the inhibition of the digestion of dietary proteins, later one having negative impacts on the flavor perception. Even though positive impacts are reported, some authors also point out that certain polyphenolic compounds can difficult the digestion of plant proteins and further impact the digestive mucosa
.
Some examples of astringent foods are red wine, tea, chocolate, and a variety of fruits and nuts. In the case of red wine, an extremely consumed drink in the world, a balanced level of astringency, to make it a desirable product, is required. By wine writers, astringency adds flavors to red wines and extends the finish. Indeed, the renowned winemaker Emile Peynaud states that the effects of harmony, balance, and elegance of astringency, correspond to great red wines
. The astringent feeling can also be perceived in some dairy products such as milk, cream, cheese, and butter. In particular, the astringency present in food and beverages containing whey proteins raises some concerns
[23]
. The incorporation of whey proteins in foods tends to increase the perception of astringency in the oral cavity, degrading the sensation of food quality
[24]
. Several other compounds may cause oral astringency, including organic and inorganic acids (such as malic or hydrochloric acid), dehydrating agents (e.g., ethanol), multivalent salts (such as potassium ammonium sulfate), and proteins. These compounds exhibit a high isoelectric point and amine-functionalized polymers, which carry positive charges at physiological pH, causing a sensation on admission to the mouth
[25]
. In the case of fruits, astringency is mainly caused by unripeness.
1.4. Influence of Astringency on Oral Perception
2.4. Influence of Astringency on Oral Perception
The complexity of astringency hinders its individualization from other oral sensations. For instance, Lee and Lawless suggested that astringency and bitterness could be confused since certain compounds can induce both
[26]. Another study compared the astringency and bitterness intensities of caffeine (bitter), quinine (astringent), and wine (astringent) for up to 120 s. Both perceptions developed similarly, slowly, and possessed lingering aftertastes. The time-courses of "dry", "rough", and astringent sensations were suitable. However, when compounds acknowledged as astringent were used, they elicited different time-courses of bitterness and astringency. On top of that, the perception of astringency needed some time to develop fully, i.e., about 15 s. It could extend for far longer, i.e., about 5 min
. Another study compared the astringency and bitterness intensities of caffeine (bitter), quinine (astringent), and wine (astringent) for up to 120 s. Both perceptions developed similarly, slowly, and possessed lingering aftertastes. The time-courses of “dry”, “rough”, and astringent sensations were suitable. However, when compounds acknowledged as astringent were used, they elicited different time-courses of bitterness and astringency. On top of that, the perception of astringency needed some time to develop fully, i.e., about 15 s. It could extend for far longer, i.e., about 5 min
[27]
.
1.5. Regulatory Factors of Oral Astringency Perception
2.5. Regulatory Factors of Oral Astringency Perception
Several factors influence the perception of astringencies, such as saliva composition, oral pH and temperature, surface properties of the oral cavity, and composition in the oral fluid (e.g., viscosity).
2. Techniques to Quantify Astringency
3. Techniques to Quantify Astringency
Currently, there is no technique able to replicate the whole complexity and accurately quantify the sequence of events involved in the oral development of astringency. A wide array of different techniques is needed to cover the behavior of the individual components and their interactions, to further correlate with the food properties.
Astringency has been measured with direct (e.g., Time-Intensity Sensory Evaluation, Descriptive Sensory Analysis and Animal Preference) and indirect methodologies (e.g., SDS-Page Based Method, Surface Plasmon Resonance and Molecular Imprinted Polymers, Protein Precipitation Methods, Hyperspectral Imaging, One-Component Model Approach, Quartz Crystal Microbalance, Cyclic Voltammetric Response and Biotribology) and by simulating molecular interactions of compounds of interests. Some of them are briefly introduced in the following sections.
2.1. Direct Methodologies
3.1. Direct Methodologies
Textural and sensorial evaluation is often an essential step in developing new food products and optimizing processing techniques. Currently, sensory analysis is one of the most used methods to evaluate astringency. The sensory analysis uses the human senses, i.e., vision, smell, touch, taste, and hearing, to assess the attributes of a product and measure human responses to foods
[28]
.
2.2. Indirect methodologies:
3.2. Indirect methodologies:
2.2.1. Biotribological Assessment
3.2.1. Biotribological Assessment
The concept of tribology was enunciated in 1966 by the Department of Education and Science in the UK
[29]
. It is an interdisciplinary science and technology known for studying the friction, wear, and lubrication between two moving surfaces/objects
[30]
. For intake and sensory perception, the behavior of interacting surfaces includes, as well, relative motions that play a crucial role in the mouth. Tongue-palate and tongue-food are perhaps the two most crucial interfaces
. The movements generate a friction/lubrication sensation between the palate and tongue, with the food product (or food–saliva mixture) acting as the lubricant with specific rheological properties
[32]
. Oral and other tribological processes related to biological systems were responsible for the advent of a new branch of tribology, rightly coined as biotribology. Biotribological studies have been giving insight into factors that affect oral sensory perception, including texture, taste, mouthfeel, and flavor
[33]
. Specifically, a better understanding of astringency development in the oral cavity may lead to advancements in the comprehension of the mechanisms that can be represented by tribological characterization.
2.2.2. Simulation by Molecular Dynamics
3.2.2. Simulation by Molecular Dynamics
Through the years, many methods, such as nuclear magnetic resonance (NMR) and X-ray diffraction (XRD), have been extensively used to understand protein structure and the relation to its functionality. However, to fully understand the mechanism of the interactions between processing conditions and proteins, it is increasingly important to explore and comprehend the effect on properties at the molecular or even atomic level
[34]
.
2.3. Other Techniques
3.3. Other Techniques
Other techniques, i.e., nuclear magnetic resonance, mass spectrometry and synchrotron radiation, atomic force microscopy and immunocytochemistry can be used to analyse properties related with astringency, such as molecular conformation, topograhy and morphology.
3. Conclusion
4. Conclusion
Oral astringency is a very complex phenomenon, which has received some research attention, nonetheless, lacking further developments to achieve a proper understanding. Astringency perception arises from the signals resulting from the interaction of astringents with the oral cavity, e.g., saliva, cell membrane proteins, epithelial cells, mechano- and chemoreceptors, that rely on the individual’s characteristics (e.g., age, saliva production, and diet). The brain interprets those signals in conjugation with another simultaneous occurring mouthfeel that can influence the perception. Therefore, many mechanisms, beyond simple lubrication, drive this mouthfeel.
A considerable amount of compounds provoke it (e.g., polyphenols, metals, some proteins, dehydrating agents, and multivalent salts), and a multitude of factors influence the oral perception (e.g., pH, viscosity, temperature, and saliva). Astringency is approached at many different levels of methods, in particular: direct (e.g., by sensory analysis and animal preference tests), indirect detection of salivary complexes (e.g., by protein precipitation and SDS-PAGE) or monitoring the interaction (e.g., QCM-D), analyzing the lubrication alteration (e.g., by oral tribology) or predicting compounds interactions (e.g., by molecular dynamics). They are well documented and accessible, with some gaps still existing; nonetheless, they are capable of quantifying the perceived astringency or some property that can be correlated with it.
Research limitations have left space to future guidelines that could ensure that consumer expectations are in agreement with the sensory experience of the product consumed. Moreover, indirect methods that could accurately and quickly predict the mouthfeel to aid the design of foods and their personalization for particular consumer groups are still needed.
References
- Engelen, L.; van der Bilt, A. Oral physiology and texture perception of semisolids. J. Texture Stud. 2007, 39, 83–113.
- Bajec, M.R.; Pickering, G.J. Astringency: Mechanisms and Perception Astringency. Food Sci. Nutr. 2008, 8398.
- Jiang, Y.; Gong, N.N.; Matsunami, H. Astringency: A More Stringent Definition. Chem. Senses 2014, 467–469.
- Bate-Smith, E.C. Flavonoid compounds in foods. Adv. Food Res. 1954, 5, 261–300.
- Joslyn, M.A.; Goldstein, J.I. Astringency of fruits and fruit products in relation to phenolic content. Adv. Food Res. 1962.
- Lawless, H.T.; Corrigan, C.J.; Lee, C.B. Interactions of astringent substances. Chem. Senses 1994, 19, 141–154.
- Peleg, H.; Bodine, K.K.; Noble, A.C.; Morrisette, C.; Dan, M. The Influence of Acid on Astringency of Alum and Phenolic Compounds. Chem. Senses 1998, 371–378.
- ASTM Standard definitions of terms relating to sensory evaluation of materials and products. In Annual Book of ASTM Standards; ASTM International: Philadelphia, PA, USA, 2004.
- Rossetti, D.; Yakubov, G.E.; Stokes, J.R.; Williamson, A.; Fuller, G.G. Interaction of human whole saliva and astringent dietary compounds investigated by interfacial shear rheology. Food Hydrocoll. 2008, 22, 1068–1078.
- Chen, J.; Stokes, J.R. Rheology and tribology: Two distinctive regimes of food texture sensation. Trends Food Sci. Technol. 2012, 25, 4–12.
- Pradal, C.; Stokes, J.R. Oral tribology: Bridging the gap between physical measurements and sensory experience. Curr. Opin. Food Sci. 2016, 9, 34–41.
- Ranc, H.; Elkhyat, A.; Servais, C.; Mac-Mary, S.; Launay, B.; Humbert, P. Friction coefficient and wettability of oral mucosal tissue: Changes induced by a salivary layer. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 155–161.
- Gibbins, H.L.; Carpenter, G.H. Alternative mechanisms of astringency—What is the role of Saliva? J. Texture Stud. 2013, 44, 364–375.
- Ployon, S.; Morzel, M.; Belloir, C.; Bonnotte, A.; Bourillot, E.; Briand, L.; Lesniewska, E.; Lherminier, J.; Aybeke, E.; Canon, F. Mechanisms of astringency: Structural alteration of the oral mucosal pellicle by dietary tannins and protective effect of bPRPs. Food Chem. 2018, 253, 79–87.
- De Wijk, R.A.; Prinz, J.F. Mechanisms underlying the role of friction in oral texture. J. Texture Stud. 2006, 37, 413–427.
- Lee, C.A.; Ismail, B.; Vickers, Z.M. The Role of Salivary Proteins in the Mechanism of Astringency. Food Sci. 2012, 77.
- Vardhanabhuti, B.; Kelly, M.A.; Luck, P.J.; Drake, M.A.; Foegeding, E.A. Roles of charge interactions on astringency of whey proteins at low pH. J. Dairy Sci. 2010, 93, 1890–1899.
- Luck, G.; Liao, H.; Murray, N.J.; Grimer, H.R.; Warminski, E.E.; Williamson, M.P.; Haslams, E.; Lilley, T.H. Polyphenols, astringency and proline-rich proteins. Phytochemistry 1994, 37, 357–371.
- Gambuti, A.; Rinaldi, A.; Pessina, R.; Moio, L. Evaluation of aglianico grape skin and seed polyphenol astringency by SDS–PAGE electrophoresis of salivary proteins after the binding reaction. Food Chem. 2006, 97, 614–620.
- Valentova, H.; Panovska, Z. SENSORY EVALUATION| Taste. In Encyclopedia of Food Sciences and Nutrition; Academic Press: San Diego, CA, USA, 2003.
- Kumari, M.; Jain, S. Tannins: An Antinutrient with Positive Effect to Manage Diabetes. Res. J. Recent Sci. 2015.
- Brandão, E.J.F.A. Development of New Formulations Based on Polysaccharides to Reduce the Astringency of Beverages; Universidade do Porto: Porto, Portugal, 2018.
- Childs, J.L.; Drake, M. Consumer Perception of Astringency in Clear Acidic Whey Protein Beverages. J. Food Sci. 2010, 75.
- Lee, C.A.; Vickers, Z.M. The astringency of whey protein beverages is caused by their acidity. Int. Dairy J. 2008, 18, 1153–1156.
- Biegler, M.; Delius, J.; Käsdorf, B.T.; Hofmann, T.; Lieleg, O. Cationic astringents alter the tribological and rheological properties of human saliva and salivary mucin solutions. Biotribology 2016, 6, 12–20.
- Lee, C.B.; Lawless, H.T. Time-course of astringent sensations. Chem. Senses 1991, 16, 225–238.
- Guinard, J.; Pangborn, R.M.; Lewis, M.J. Preliminary studies on acidity-astringency interactions in model solutions and wines. J. Sci. Food Agric. 1986, 37, 811–817.
- Drake, M.A. Invited review: Sensory analysis of dairy foods. J. Dairy Sci. 2007, 90, 4925–4937.
- Jost, H.P. Tribology—Origin and future. Wear 1990, 136.
- Bhushan, B. Principles and Applications of Tribology; John Wiley & Sons: Colombus, OH, USA, 1999.
- Upadhyay, R.; Brossard, N.; Chen, J. Mechanisms underlying astringency: Introduction to an oral tribology approach. J. Phys. D Appl. Phys. 2016, 49, 104003.
- Prakash, S.; Dan, D.; Tan, Y.; Chen, J. Applications of tribology in studying food oral processing and texture perception. FRIN 2013, 54, 1627–1635.
- Selway, N.; Stokes, J.R. Soft Materials Deformation, Flow, and Lubrication Between Compliant Substrates: Impact on Flow Behavior, Mouthfeel, Stability, and Flavor. Annu. Rev. Food Sci. Technol. 2014.
- Singh, A.; Vanga, S.K.; Orsat, V.; Raghavan, V. Application of molecular dynamic simulation to study food proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2779–2789.
