Visual plasticity is classically considered to occur essentially in the primary and secondary cortical areas. Subcortical visual areas such as the dorsal lateral geniculate nucleus (dLGN) or the superior colliculus (SC) have long been held as basic structures responsible for a stable and defined function. In this model, the dLGN was considered as a relay of visual information travelling from the retina to cortical areas and the SC as a sensory integrator orienting body movements towards visual targets. However, recent findings suggest that both dLGN and SC neurons express functional plasticity, adding unexplored layers of complexity to their previously attributed functions. The existence of neuronal plasticity at the level of visual subcortical areas redefines our approach of the visual system.
- superior colliculus
- synaptic plasticity
- lateral geniculate nucleus
- visual system
- functional plasticity
1. Introduction
1.1. Lateral Geniculate Nucleus and Superior Colliculus
1.2. Cortical and Subcortical Plasticity
2. Functional Plasticity in Subcortical Visual Areas
2.1. Functional Plasticity in the dLGN
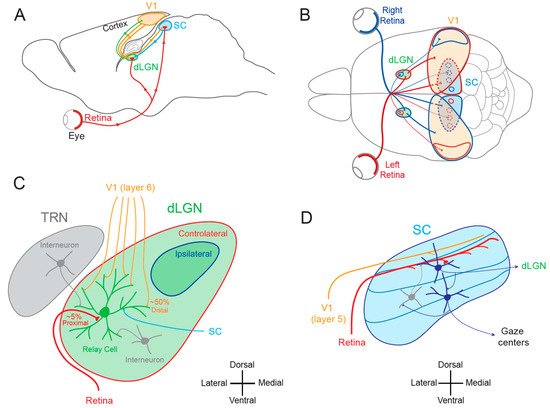
The notion that dLGN neurons do not express plasticity was disproved a decade after Wiesel and Hubel’s publication. Indeed, Ikeda and Wright showed in 1976 that the spatial resolution of dLGN neurons activated by the deviating eye in kittens reared with a squint is considerably reduced compared to that of neurons activated by the normal eye [28][18]. This result was the first to suggest that loss of normal binocular vision leads to plastic changes in the LGN. Later on, it was shown in amblyopic patients that functional deficits in visual response are already observed at the stage of the dLGN [29][19]. In addition, in contrast to what was previously assumed, about half of the rodent dLGN relay neurons in a given monocular territory in fact receive inputs from both eyes, indicating a potential binocularity for a large proportion of dLGN neurons [30,31,32,33,34,35,36][20][21][22][23][24][25][26]. Moreover, spatial receptive fields at eye opening in mouse dLGN are ~2 times larger than in adulthood due to an increase in surround suppression owing to an increased in feed-forward inhibition [37][27]. Furthermore, monocular deprivation (MD) in the mouse has been shown to produce a large shift in ocular dominance (OD) in dLGN neurons (Figure 2A) [38,39,40][28][29][30]. In one of these studies, GABAergic synaptic inhibition was found to be critical [39][29]. It is very unlikely that the plasticity observed in the dLGN only represents altered feedback from the cortex, because the shift in dLGN responses was resistant to cortical inactivation using the GABAA receptor agonist muscimol [38][28].
2.2. Functional Plasticity in the SC
2.2. Functional Plasticity in the SC
The SC is the mammalian equivalent of the optic tectum in inferior vertebrates [5]. While many studies reported functional and synaptic plasticity in the tadpole optic tectum [43[33][34][35][36],44,45,46], fewer investigations have been performed on the mammalian SC. As for the dLGN, the SC was thought to be largely devoid of functional plasticity, since receptive field features were found unchanged after MD [47][37]. However, recent findings suggest that SC express functional plasticity. The best demonstration of SC plasticity comes from studies on hemianopia, a permanent visual deficit caused by cortical trauma [41,42,48,49][31][32][38][39]. Patients with unilateral hemianopia are totally blind in the contralateral visual hemi-field but have preserved subcortical visual structures such as the SC. In basic post-traumatic conditions, hemianopia patients display a total lack of gaze orientation towards the blind hemi-field; a behavioral response depending on cortico-collicular connections. However, when the visual stimulus was temporally paired with an auditory stimulus occurring in the same region of the visual space (i.e., audio-visual training), normal gaze orientation towards the blind hemi-field (Figure 2B) was observed both in patients [41][31] and cats [42,49][32][39]. Interestingly, the re-emergence of visual behavior in cats is correlated with the reinstatement of visual responsiveness in deep layer neurons of the ipsilesional SC [42][32]. This audio-visual training procedure is thought to be related to the Hebbian learning and to reflect potentiation of visually activated synapses onto gaze-orientation related premotor neurons within the SC that fired under the conjoint activation of auditory synapses. In fact, audio-visual training has been shown to be able to reveal auditory or visual responses that were absent initially [50][40].
3. Structural Plasticity in Subcortical Visual Areas
3.1. Structural Plasticity in the dLGN
3.2. Structural Plasticity in the SC
4. Synaptic Plasticity in Subcortical Visual Areas
4.1. Synaptic Plasticity in the dLGN
4.1.1. Hebbian Synaptic Plasticity in the dLGN
4.1.2. Homeostatic Synaptic Plasticity in the dLGN
4.2. Synaptic Plasticity in the SC
4.2.1. Hebbian Synaptic Plasticity in the SC
4.2.2. Homeostatic Synaptic Plasticity in the SC
5. Intrinsic Plasticity in Subcortical Visual Areas
Beyond synaptic plasticity, changes in intrinsic neuronal excitability represent the other side of functional plasticity that usually goes hand-in-hand with synaptic modifications [104,105][75][76] and possibly participate to developmental plasticity and learning [106,107][77][78]. Intrinsic plasticity is generally triggered by synaptic activity (induction phase) that induces plasticity of synaptic transmission in parallel. However, the expression of plasticity of intrinsic neuronal excitability depends on the regulation of voltage-gated ion channels (expression phase) such as hyperpolarization-activated cyclic nucleotide-gated (HCN) channels [108[79][80][81][82],109,110,111], Nav [112][83], Kv1 [113][84] and Kv7 [114][85] channels.
6. Conclusions
References
- Weyand, T.G. The Multifunctional Lateral Geniculate Nucleus. Rev. Neurosci. 2016, 27, 135–157.
- Sherman, S.M. Thalamus Plays a Central Role in Ongoing Cortical Functioning. Nat. Neurosci. 2016, 19, 533–541.
- Ghodrati, M.; Khaligh-Razavi, S.-M.; Lehky, S.R. Towards Building a More Complex View of the Lateral Geniculate Nucleus: Recent Advances in Understanding Its Role. Prog. Neurobiol. 2017, 156, 214–255.
- Basso, M.A.; Bickford, M.E.; Cang, J. Unraveling Circuits of Visual Perception and Cognition through the Superior Colliculus. Neuron 2021, 109, 918–937.
- Isa, T.; Marquez-Legorreta, E.; Grillner, S.; Scott, E.K. The Tectum/Superior Colliculus as the Vertebrate Solution for Spatial Sensory Integration and Action. Curr. Biol. 2021, 31, R741–R762.
- Cooper, B.; McPeek, R.M. Role of the Superior Colliculus in Guiding Movements Not Made by the Eyes. Annu. Rev. Vis Sci 2021.
- Ito, S.; Feldheim, D.A. The Mouse Superior Colliculus: An Emerging Model for Studying Circuit Formation and Function. Front. Neural Circuits 2018, 12, 10.
- Denman, D.J.; Contreras, D. On Parallel Streams through the Mouse Dorsal Lateral Geniculate Nucleus. Front. Neural Circuits 2016, 10, 20.
- Sherman, S.M.; Wilson, J.R.; Kaas, J.H.; Webb, S.V. X- and Y-Cells in the Dorsal Lateral Geniculate Nucleus of the Owl Monkey (Aotus Trivirgatus). Science 1976, 192, 475–477.
- Dreher, B.; Fukada, Y.; Rodieck, R.W. Identification, Classification and Anatomical Segregation of Cells with X-like and Y-like Properties in the Lateral Geniculate Nucleus of Old-World Primates. J. Physiol. 1976, 258, 433–452.
- Lee, P.H.; Sooksawate, T.; Yanagawa, Y.; Isa, K.; Isa, T.; Hall, W.C. Identity of a Pathway for Saccadic Suppression. Proc. Natl. Acad. Sci. USA 2007, 104, 6824–6827.
- Hooks, B.M.; Chen, C. Circuitry Underlying Experience-Dependent Plasticity in the Mouse Visual System. Neuron 2020, 106, 21–36.
- Wiesel, T.N.; Hubel, D.H. Effect of Visual Deprivation on Morphology and Physiology of Cells in the Cat’s Lateral Geniculate Body. J. Neurophysiol. 1963, 26, 978–993.
- Derrington, A.M.; Hawken, M.J. Spatial and Temporal Properties of Cat Geniculate Neurones after Prolonged Deprivation. J. Physiol. 1981, 314, 107–120.
- Blakemore, C.; Vital-Durand, F. Effects of Visual Deprivation on the Development of the Monkey’s Lateral Geniculate Nucleus. J. Physiol. 1986, 380, 493–511.
- Levitt, J.B.; Schumer, R.A.; Sherman, S.M.; Spear, P.D.; Movshon, J.A. Visual Response Properties of Neurons in the LGN of Normally Reared and Visually Deprived Macaque Monkeys. J. Neurophysiol. 2001, 85, 2111–2129.
- Halassa, M.M.; Sherman, S.M. Thalamocortical Circuit Motifs: A General Framework. Neuron 2019, 103, 762–770.
- Ikeda, H.; Wright, M.J. Properties of LGN Cells in Kittens Reared with Convergent Squint: A Neurophysiological Demonstration of Amblyopia. Exp. Brain Res. 1976, 25, 63–77.
- Hess, R.F.; Thompson, B.; Gole, G.; Mullen, K.T. Deficient Responses from the Lateral Geniculate Nucleus in Humans with Amblyopia. Eur. J. Neurosci. 2009, 29, 1064–1070.
- Hammer, S.; Monavarfeshani, A.; Lemon, T.; Su, J.; Fox, M.A. Multiple Retinal Axons Converge onto Relay Cells in the Adult Mouse Thalamus. Cell. Rep. 2015, 12, 1575–1583.
- Morgan, J.L.; Berger, D.R.; Wetzel, A.W.; Lichtman, J.W. The Fuzzy Logic of Network Connectivity in Mouse Visual Thalamus. Cell 2016, 165, 192–206.
- Rompani, S.B.; Müllner, F.E.; Wanner, A.; Zhang, C.; Roth, C.N.; Yonehara, K.; Roska, B. Different Modes of Visual Integration in the Lateral Geniculate Nucleus Revealed by Single-Cell-Initiated Transsynaptic Tracing. Neuron 2017, 93, 767–776.e6.
- Zeater, N.; Cheong, S.K.; Solomon, S.G.; Dreher, B.; Martin, P.R. Binocular Visual Responses in the Primate Lateral Geniculate Nucleus. Curr. Biol. 2015, 25, 3190–3195.
- Howarth, M.; Walmsley, L.; Brown, T.M. Binocular Integration in the Mouse Lateral Geniculate Nuclei. Curr. Biol. 2014, 24, 1241–1247.
- Huh, C.Y.L.; Abdelaal, K.; Salinas, K.J.; Gu, D.; Zeitoun, J.; Figueroa Velez, D.X.; Peach, J.P.; Fowlkes, C.C.; Gandhi, S.P. Long-Term Monocular Deprivation during Juvenile Critical Period Disrupts Binocular Integration in Mouse Visual Thalamus. J. Neurosci. 2020, 40, 585–604.
- Litvina, E.Y.; Chen, C. Functional Convergence at the Retinogeniculate Synapse. Neuron 2017, 96, 330–338.e5.
- Tschetter, W.W.; Govindaiah, G.; Etherington, I.M.; Guido, W.; Niell, C.M. Refinement of Spatial Receptive Fields in the Developing Mouse Lateral Geniculate Nucleus Is Coordinated with Excitatory and Inhibitory Remodeling. J. Neurosci. 2018, 38, 4531–4542.
- Jaepel, J.; Hübener, M.; Bonhoeffer, T.; Rose, T. Lateral Geniculate Neurons Projecting to Primary Visual Cortex Show Ocular Dominance Plasticity in Adult Mice. Nat. Neurosci. 2017, 20, 1708–1714.
- Sommeijer, J.-P.; Ahmadlou, M.; Saiepour, M.H.; Seignette, K.; Min, R.; Heimel, J.A.; Levelt, C.N. Thalamic Inhibition Regulates Critical-Period Plasticity in Visual Cortex and Thalamus. Nat. Neurosci. 2017, 20, 1715–1721.
- Rose, T.; Bonhoeffer, T. Experience-Dependent Plasticity in the Lateral Geniculate Nucleus. Curr. Opin. Neurobiol. 2018, 53, 22–28.
- Bolognini, N.; Rasi, F.; Coccia, M.; Làdavas, E. Visual Search Improvement in Hemianopic Patients after Audio-Visual Stimulation. Brain 2005, 128, 2830–2842.
- Jiang, H.; Stein, B.E.; McHaffie, J.G. Multisensory Training Reverses Midbrain Lesion-Induced Changes and Ameliorates Haemianopia. Nat. Commun. 2015, 6, 7263.
- Zhang, L.I.; Tao, H.W.; Poo, M. Visual Input Induces Long-Term Potentiation of Developing Retinotectal Synapses. Nat. Neurosci. 2000, 3, 708–715.
- Tao, H.W.; Zhang, L.I.; Engert, F.; Poo, M. Emergence of Input Specificity of Ltp during Development of Retinotectal Connections In Vivo. Neuron 2001, 31, 569–580.
- Lien, C.-C.; Mu, Y.; Vargas-Caballero, M.; Poo, M. Visual Stimuli-Induced LTD of GABAergic Synapses Mediated by Presynaptic NMDA Receptors. Nat. Neurosci. 2006, 9, 372–380.
- Van Rheede, J.J.; Richards, B.A.; Akerman, C.J. Sensory-Evoked Spiking Behavior Emerges via an Experience-Dependent Plasticity Mechanism. Neuron 2015, 87, 1050–1062.
- Hoffmann, K.P.; Sherman, S.M. Effects of Early Monocular Deprivation on Visual Input to Cat Superior Colliculus. J. Neurophysiol. 1974, 37, 1276–1286.
- Stein, B.E.; Rowland, B.A. Using Superior Colliculus Principles of Multisensory Integration to Reverse Hemianopia. Neuropsychologia 2020, 141, 107413.
- Dakos, A.S.; Jiang, H.; Stein, B.E.; Rowland, B.A. Using the Principles of Multisensory Integration to Reverse Hemianopia. Cereb. Cortex 2020, 30, 2030–2041.
- Yu, L.; Rowland, B.A.; Xu, J.; Stein, B.E. Multisensory Plasticity in Adulthood: Cross-Modal Experience Enhances Neuronal Excitability and Exposes Silent Inputs. J. Neurophysiol. 2013, 109, 464–474.
- Chen, C.; Regehr, W.G. Developmental Remodeling of the Retinogeniculate Synapse. Neuron 2000, 28, 955–966.
- Jaubert-Miazza, L.; Green, E.; Lo, F.-S.; Bui, K.; Mills, J.; Guido, W. Structural and Functional Composition of the Developing Retinogeniculate Pathway in the Mouse. Vis. Neurosci. 2005, 22, 661–676.
- Hong, Y.K.; Park, S.; Litvina, E.Y.; Morales, J.; Sanes, J.R.; Chen, C. Refinement of the Retinogeniculate Synapse by Bouton Clustering. Neuron 2014, 84, 332–339.
- Krahe, T.E.; El-Danaf, R.N.; Dilger, E.K.; Henderson, S.C.; Guido, W. Morphologically Distinct Classes of Relay Cells Exhibit Regional Preferences in the Dorsal Lateral Geniculate Nucleus of the Mouse. J. Neurosci. 2011, 31, 17437–17448.
- Charalambakis, N.E.; Govindaiah, G.; Campbell, P.W.; Guido, W. Developmental Remodeling of Thalamic Interneurons Requires Retinal Signaling. J. Neurosci. 2019, 39, 3856–3866.
- Butts, D.A.; Kanold, P.O.; Shatz, C.J. A Burst-Based “Hebbian” Learning Rule at Retinogeniculate Synapses Links Retinal Waves to Activity-Dependent Refinement. PLoS Biol. 2007, 5, e61.
- Krahe, T.E.; Guido, W. Homeostatic Plasticity in the Visual Thalamus by Monocular Deprivation. J. Neurosci. 2011, 31, 6842–6849.
- O’Leary, D.D.; Wilkinson, D.G. Eph Receptors and Ephrins in Neural Development. Curr. Opin. Neurobiol. 1999, 9, 65–73.
- Finlay, B.L.; Schneps, S.E.; Schneider, G.E. Orderly Compression of the Retinotectal Projection Following Partial Tectal Ablation in the Newborn Hamster. Nature 1979, 280, 153–155.
- Frost, D.O.; Schneider, G.E. Plasticity of Retinofugal Projections after Partial Lesions of the Retina in Newborn Syrian Hamsters. J. Comp. Neurol. 1979, 185, 517–567.
- Simon, D.K.; O’Leary, D.D. Responses of Retinal Axons in Vivo and in Vitro to Position-Encoding Molecules in the Embryonic Superior Colliculus. Neuron 1992, 9, 977–989.
- Torborg, C.L.; Feller, M.B. Spontaneous Patterned Retinal Activity and the Refinement of Retinal Projections. Prog. Neurobiol. 2005, 76, 213–235.
- Bickford, M.E.; Slusarczyk, A.; Dilger, E.K.; Krahe, T.E.; Kucuk, C.; Guido, W. Synaptic Development of the Mouse Dorsal Lateral Geniculate Nucleus. J. Comp. Neurol. 2010, 518, 622–635.
- Hong, Y.K.; Chen, C. Wiring and Rewiring of the Retinogeniculate Synapse. Curr. Opin. Neurobiol. 2011, 21, 228–237.
- Guido, W. Development, Form, and Function of the Mouse Visual Thalamus. J. Neurophysiol. 2018, 120, 211–225.
- Gustafsson, B.; Wigström, H.; Abraham, W.C.; Huang, Y.Y. Long-Term Potentiation in the Hippocampus Using Depolarizing Current Pulses as the Conditioning Stimulus to Single Volley Synaptic Potentials. J. Neurosci. 1987, 7, 774–780.
- Debanne, D.; Gähwiler, B.H.; Thompson, S.M. Asynchronous Pre- and Postsynaptic Activity Induces Associative Long-Term Depression in Area CA1 of the Rat Hippocampus in Vitro. Proc. Natl. Acad. Sci. USA 1994, 91, 1148–1152.
- Changeux, J.P.; Danchin, A. Selective Stabilisation of Developing Synapses as a Mechanism for the Specification of Neuronal Networks. Nature 1976, 264, 705–712.
- Corriveau, R.A.; Huh, G.S.; Shatz, C.J. Regulation of Class I MHC Gene Expression in the Developing and Mature CNS by Neural Activity. Neuron 1998, 21, 505–520.
- Huh, G.S.; Boulanger, L.M.; Du, H.; Riquelme, P.A.; Brotz, T.M.; Shatz, C.J. Functional Requirement for Class I MHC in CNS Development and Plasticity. Science 2000, 290, 2155–2159.
- Goddard, C.A.; Butts, D.A.; Shatz, C.J. Regulation of CNS Synapses by Neuronal MHC Class, I. Proc. Natl. Acad. Sci. USA 2007, 104, 6828–6833.
- Needleman, L.A.; Liu, X.-B.; El-Sabeawy, F.; Jones, E.G.; McAllister, A.K. MHC Class I Molecules Are Present Both Pre- and Postsynaptically in the Visual Cortex during Postnatal Development and in Adulthood. Proc. Natl. Acad. Sci. USA 2010, 107, 16999–17004.
- Elmer, B.M.; McAllister, A.K. Major Histocompatibility Complex Class I Proteins in Brain Development and Plasticity. Trends Neurosci. 2012, 35, 660–670.
- Lee, H.; Brott, B.K.; Kirkby, L.A.; Adelson, J.D.; Cheng, S.; Feller, M.B.; Datwani, A.; Shatz, C.J. Synapse Elimination and Learning Rules Co-Regulated by MHC Class I H2-Db. Nature 2014, 509, 195–200.
- Zhang, L.I.; Tao, H.W.; Holt, C.E.; Harris, W.A.; Poo, M. A Critical Window for Cooperation and Competition among Developing Retinotectal Synapses. Nature 1998, 395, 37–44.
- Vislay-Meltzer, R.L.; Kampff, A.R.; Engert, F. Spatiotemporal Specificity of Neuronal Activity Directs the Modification of Receptive Fields in the Developing Retinotectal System. Neuron 2006, 50, 101–114.
- Okada, Y.; Miyamoto, T. Formation of Long-Term Potentiation in Superior Colliculus Slices from the Guinea Pig. Neurosci. Lett. 1989, 96, 108–113.
- Okada, Y. The Properties of the Long-Term Potentiation (LTP) in the Superior Colliculus. Prog. Brain Res. 1993, 95, 287–296.
- Zhao, J.-P.; Phillips, M.A.; Constantine-Paton, M. Long-Term Potentiation in the Juvenile Superior Colliculus Requires Simultaneous Activation of NMDA Receptors and L-Type Ca2+ Channels and Reflects Addition of Newly Functional Synapses. J. Neurosci. 2006, 26, 12647–12655.
- Lu, W.; Constantine-Paton, M. Eye Opening Rapidly Induces Synaptic Potentiation and Refinement. Neuron 2004, 43, 237–249.
- Zhao, J.-P.; Murata, Y.; Constantine-Paton, M. Eye Opening and PSD95 Are Required for Long-Term Potentiation in Developing Superior Colliculus. Proc. Natl. Acad. Sci. USA 2013, 110, 707–712.
- Lo, F.-S.; Mize, R.R. Properties of LTD and LTP of Retinocollicular Synaptic Transmission in the Developing Rat Superior Colliculus. Eur. J. Neurosci. 2002, 15, 1421–1432.
- Van Keuren-Jensen, K.; Cline, H.T. Visual Experience Regulates Metabotropic Glutamate Receptor-Mediated Plasticity of AMPA Receptor Synaptic Transmission by Homer1a Induction. J. Neurosci. 2006, 26, 7575–7580.
- Deeg, K.E.; Aizenman, C.D. Sensory Modality-Specific Homeostatic Plasticity in the Developing Optic Tectum. Nat. Neurosci. 2011, 14, 548–550.
- Debanne, D.; Inglebert, Y.; Russier, M. Plasticity of Intrinsic Neuronal Excitability. Curr. Opin. Neurobiol. 2019, 54, 73–82.
- Debanne, D.; Russier, M. The Contribution of Ion Channels in Input-Output Plasticity. Neurobiol. Learn Mem. 2019, 166, 107095.
- Daoudal, G.; Debanne, D. Long-Term Plasticity of Intrinsic Excitability: Learning Rules and Mechanisms. Learn. Mem. 2003, 10, 456–465.
- Titley, H.K.; Brunel, N.; Hansel, C. Toward a Neurocentric View of Learning. Neuron 2017, 95, 19–32.
- Fan, Y.; Fricker, D.; Brager, D.H.; Chen, X.; Lu, H.-C.; Chitwood, R.A.; Johnston, D. Activity-Dependent Decrease of Excitability in Rat Hippocampal Neurons through Increases in I(h). Nat. Neurosci. 2005, 8, 1542–1551.
- Brager, D.H.; Johnston, D. Plasticity of Intrinsic Excitability during Long-Term Depression Is Mediated through MGluR-Dependent Changes in I(h) in Hippocampal CA1 Pyramidal Neurons. J. Neurosci. 2007, 27, 13926–13937.
- Campanac, E.; Daoudal, G.; Ankri, N.; Debanne, D. Downregulation of Dendritic I(h) in CA1 Pyramidal Neurons after LTP. J. Neurosci. 2008, 28, 8635–8643.
- Gasselin, C.; Inglebert, Y.; Ankri, N.; Debanne, D. Plasticity of Intrinsic Excitability during LTD Is Mediated by Bidirectional Changes in H-Channel Activity. Sci. Rep. 2017, 7, 14418.
- Xu, J.; Kang, N.; Jiang, L.; Nedergaard, M.; Kang, J. Activity-Dependent Long-Term Potentiation of Intrinsic Excitability in Hippocampal CA1 Pyramidal Neurons. J. Neurosci. 2005, 25, 1750–1760.
- Campanac, E.; Gasselin, C.; Baude, A.; Rama, S.; Ankri, N.; Debanne, D. Enhanced Intrinsic Excitability in Basket Cells Maintains Excitatory-Inhibitory Balance in Hippocampal Circuits. Neuron 2013, 77, 712–722.
- Incontro, S.; Sammari, M.; Azzaz, F.; Inglebert, Y.; Ankri, N.; Russier, M.; Fantini, J.; Debanne, D. Endocannabinoids Tune Intrinsic Excitability in O-LM Interneurons by Direct Modulation of Post-Synaptic Kv7 Channels. J. Neurosci. 2021.
- Pigeat, R.; Chausson, P.; Dreyfus, F.M.; Leresche, N.; Lambert, R.C. Sleep Slow Wave-Related Homo and Heterosynaptic LTD of Intrathalamic GABAAergic Synapses: Involvement of T-Type Ca2+ Channels and Metabotropic Glutamate Receptors. J. Neurosci. 2015, 35, 64–73.
