The level of carbon dioxide in the atmosphere is growing rapidly due to fossil fuel combustion processes, heavy oil, coal, oil shelter, and exhausts from automobiles for energy generation, which lead to depletion of the ozone layer and consequently result in global warming. The realization of a carbon-neutral environment is the main focus of science and academic researchers of today. Several processes were employed to minimize carbon dioxide in the air, some of which include the utilization of non-fossil sources of energy like solar, nuclear, and biomass-based fuels. Consequently, these sources were reported to have a relatively high cost of production and maintenance. The applications of both homogeneous and heterogeneous processes in carbon capture and storage were investigated in recent years and the focus now is on the conversion of CO2 into useful chemicals and compounds. It was established that CO2 can undergo cycloaddition reaction with epoxides under the influence of special catalysts to give cyclic carbonates, which can be used as value-added chemicals at a different level of pharmaceutical and industrial applications. Among the various catalysts studied for this reaction, metal-organic frameworks are now on the frontline as a potential catalyst due to their special features and easy synthesis. Several metal-organic framework (MOF)-based catalysts were studied for their application in transforming CO2 to organic carbonates using epoxides. Here, we report some recent studies of porous MOF materials and an in-depth discussion of two repeatedly used metal-organic frameworks as a catalyst in the conversion of CO2 to organic carbonates
- cycloaddition
- epoxides
- carbon dioxide
- metal-organic frameworks
1. Reaction Mechanism for the Production of Cyclic Carbonates from CO2 and Epoxides
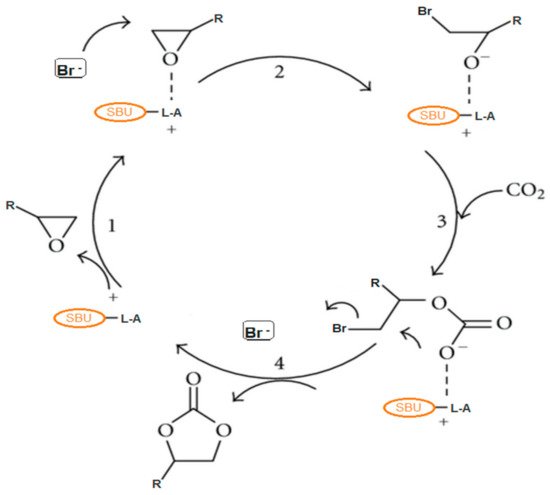
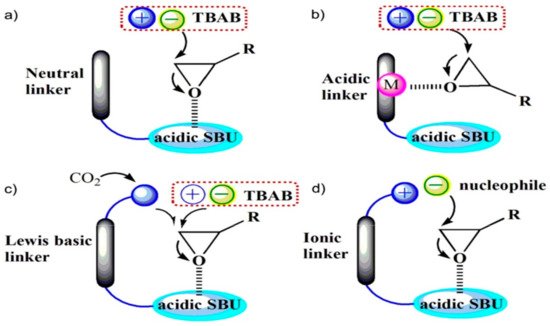
2. Metal-Organic Frameworks in CO2 Cycloaddition with Epoxides
| Entry | MOF Material | Co-Catalyst | Catalyst: Cocatalyst Loading (mol%) |
S | BET | (m | 2 | /g) | Epoxide | Press (atm) |
Temp. (°C) | Time (h) | Selectivity (%) | Yield (%) |
Isosteric Heat Q | st | (Kj/Mol) | Reusability | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Al(OH) (O | 2 | C–CH=CH–CO | 2 | )∙ | n | H | 2 | O | TBABr | 0.02:0.002 | 1169 | ECH | 10 | 50 | 6 | 97 | 95 | 23 | 4 cycles | [60] | [134] | ||||||
| 2 | Zn | 2 | (Py)(Atz) | 2 | ∙DMF∙2H | 2 | O | TBABr | 0.1:0.1 | 764.5 | PO | 15 | 60 | 4 | 98 | 92 | 27.7 | 6 cycles | [40] | [118] | ||||||||
| 3 | [In | 2 | (L)(OH) | 2 | ]·2DMF·2H | 2 | O | TBABr | 0.5:0.2 | 1022 | EBH | 1 | 70 | 12 | 89 | 99 | - | 5 cycles | [61] | [83] | ||||||||
| 4 | F-Mn-MOF-74 | TBABr | 0.1:0.031 | 20.83 | SO | 10 | 100 | 6 | 99 | 99 | - | 7 cycles | [62] | [135] | ||||||||||||||
| 5 | PCN-222(Co)@MTTB | TBABr | 0.1:0.216 | PO/ECH | 1 | 50 | 20 | 98 | >98 | - | 3 cycles | [51] | [126] | |||||||||||||||
| 6 | rho-ZMOF | TBABr | 0.1:1.4 | 871 | ECH | 10 | 40 | 3 | 98 | 98 | - | 5 cycles | [54] | [129] | ||||||||||||||
| 7 | Co-MOF-2 {[Co(BDC)(L)]·2H | 2 | O.xG}n | TBABr | 1.8:2.5 | 6.8 | SO/ECH | 1 | 40 | 12 | 99 | 99 | 35.0 | 6 cycles | [44] | [119] | ||||||||||||
| 8 | {[Zn(H | 2 | O)(HL)]⋅(DMF) | 2 | (H | 2 | O) | 2 | }n | TBABr | 0.25:0.232 | 945 | PO | 1 | RT | 48 | - | 76 | - | - | [48] | [123] | ||||||
| 9 | MOF-5-MIX | TBABr | 0.5:0.5 | 357 | ECH | 12 | 50 | 6 | 99 | 98 | - | 5 cycles | [63] | |||||||||||||||
| 10 | Ce-NU-1008 | TBABr | 0.02:0.002 | SO | 1 | RT | 20 | 95 | - | 3 cycles | [64] | [57] | ||||||||||||||||
| 11 | Co-MOF-2 {[Co(BDC)(L)]·2H | 2 | O·xG}n | KI | 5.0:0.2 | 6.8 | SEO | 1 | 40 | 8 | 99 | 99 | 35.0 | 6 cycles | [65] | [56] | ||||||||||||
| 12 | {[Ni | 3 | HL(μ3-OH)(H | 2 | O) | 2 | ]∙3(H | 2 | O)∙DMA}n | TBABr | 0.025:1.5 | 743.5 | ECH | 10 | 100 | 6 | - | >99 | - | 5 cycles | [66] | [33] | ||||||
| 13 | [(Cu | 2 | BPDSDC∙4DMF)∙2DMF]n | TBABr | 0.05:0.1 | - | PO | 25 | 80 | 5 | 98 | 99 | - | 4 cycles | [67] | [136] | ||||||||||||
| 14 | {[Co | 6 | (OH) | 2 | (H | 2 | O) | 4 | (cpt) | 9 | ](NO | 3 | )(DMF) | 13 | } | TBABr | 0.1:2 | 873 | PO | 1 | 40 | 48 | 97 | 97 | 32 | 4 cycles | [68] | [137] |
| 15 | InDCPN-Cl | TBABr | 0.05:5.00 | 997 | SO | 1 | 80 | 24 | 98 | 93 | 30 | 5 cycles | [16] | [96] | ||||||||||||||
| 16 | Ce-NU-1008 | TBABr | 0.002:0.02 | 910 | SO | 1 | RT | 20 | 95 | - | - | [64] | [57] | |||||||||||||||
| 17 | MOF-5@Imidazolium iodide | - | - | 277.9 | SO | 10 | 110 | 8 | - | 92 | - | 4 cycles | [69] | [138] | ||||||||||||||
| 18 | [(CH | 3 | ) | 2 | NH | 2 | ][M(COOH) | 3 | ] | - | 13.1 | 13.11 | PO | 20 | 120 | 6 | 100 | 98 | - | 3 cycles | [70] | [139] | ||||||
| 19 | Im-MnF [C | 3 | H | 5 | N | 2 | ][Mn(COOH) | 3 | ] | - | - | 81.57 | ECH | 15 | 100 | 6 | 99 | 95 | - | - | [71] | [140] | ||||||
| 20 | Pt/Mg-MOF-74 | - | 513 | PO | 17.5 | 150 | 4 | 77 | 44 | - | 3 cycles | [72] |
3. MIL-101 Based MOFs in CO2 Cycloaddition with Epoxides
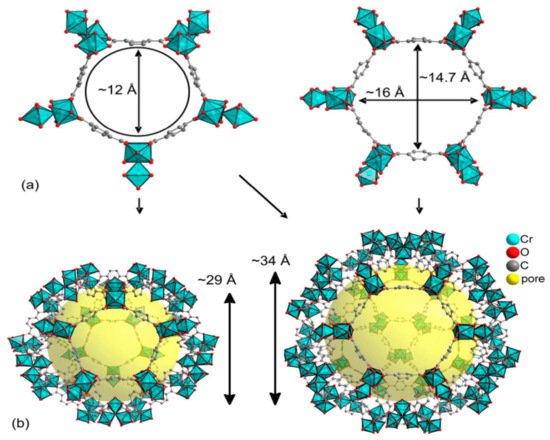
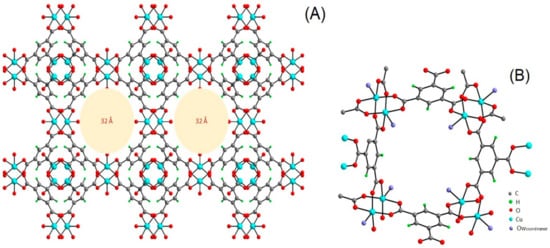
MIL-101 is one of the repeatedly reported MOF materials with a high potential catalytic activity for the conversion of CO2 to cyclic carbonates. This was ascribed to its possession of Lewis-acid sites due to Cr2+, present at the metal center [65][56] and structural flexibility, which allows its modifications by substituting different functional groups in the organic ligand but maintains the backbone structures. The synthesis and structural elucidation of MIL-101(Cr) was reported by different researchers [73][74][29][75][76][77][78][79][80][81][82][83][85,86,108,141,142,143,144,145,146,147,148,149]. MIL-101 is a three-dimensional structure based on chromium terephthalate that was first synthesized by Fėrey et al. [82][148], having the empirical formula {Cr3(OH)(H2O)2O[(O2C)C6H4(CO2]3∙nH2O} with the given name MIL: (Materials Institute Lavosier) in 2005 (Figure 58). The material has a very stable structure with excellent water resistibility even under acidic conditions and was proven to have thermal stability up to 300 °C under air. The MIL-101(Cr) structure exhibited a large surface area of approximately 4100 m2 g−1 and contained two different types of cages with diameters of 29 and 34 Å, which had pore openings of 12 and 16 Å, respectively (Figure 69) [82][84][85][148,150,151]. Those special properties made MIL-101 possess superior catalytic activity, which was applied in different applications [31][32][86][87][88][89][90][110,111,152,153,154,155,156]. The unique porosity of three-dimensional frameworks forms exclusive channels with large surface areas, which can enhance CO2 by providing sufficient reaction spaces. It also allows the encapsulation of other catalytic active materials into the large pores to improve the catalytic activity of the MIL-101 by forming a composites with enhanced activity for application in various fields [77][89][143,155]. The MOF was also applied as a catalyst in cycloaddition reaction of CO2 with epoxides as a single component catalyst without the addition of co-catalyst [59][55].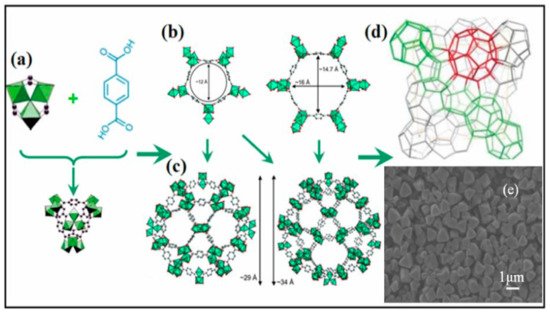
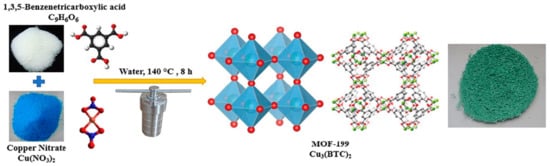
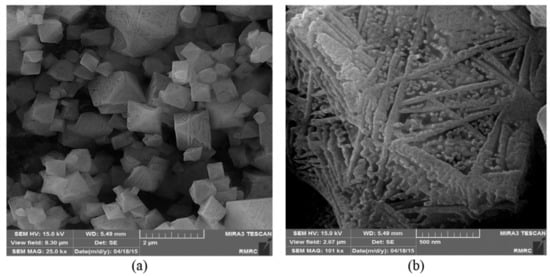
4. HKUST-1 for CO2 Cycloaddition with Epoxides
Abbreviations
| MOFs | Metal-organic frameworks |
| TBABr | Tetrabutyl ammonium bromide |
| SBU | Secondary Building Unit |
| SO | Styrene Oxide |
| PO | Propylene oxide |
| ECH | Epichlorohydrin |
| EBH | Epibromohydrin |
| SEO | Spiro-Epoxy Oxindole |
| BTC | 1,3,5-benzenetricarboxylate |
| HKUST | Hong Kong University of Science and Technology |
| RT | Room temperature |
| KI | potassium iodide |
| Å | Aperture |
