MCTs can be easily formed using non-adhesive cell culture methods such as the hanging-drop method or centrifugation of cells in a suspension culture method
[2][6][46,50], in which the cells in the suspension culture medium are located at the bottom due to the presence of a meniscus in the middle layer
[7][51]. Conventional approaches to generate cancer cell aggregation are hindered by variations in the cell number and spheroid size, high-shear force, and their labor-intensive nature
[8][52]. Recently, different microfabrication methods, including microfluidics and microwell, have exhibited the potential to form a large number of well-organized spheroids. Zhao et al., (2019) first introduced a 3D-printed hanging-drop dripper (3D-phd) device that enables long-term production of uniform cancer spheroids (
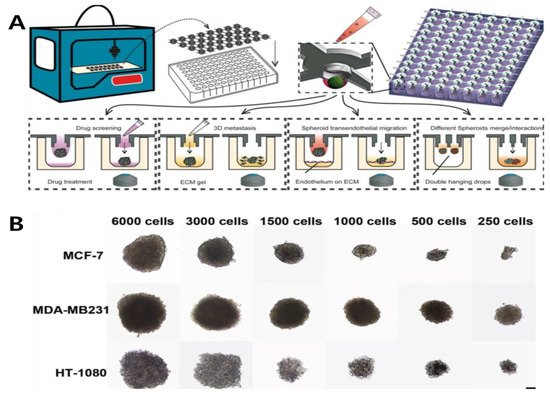
Figure 12)
[9][28]. In addition to culturing MCF-7 and MDA-MB-231 human breast cancer cells, this device performs a series of biomedical assessments, including imaging analysis, gene expression mapping, and anticancer drug assays. They proved that this device has some merits such as ease of design to perform 3D tumor migration analysis and can extend to organ-on-chip engineering, as well as assessing the anticancer drug efficiency on the co-culture spheroids. Some use the microfluidic technology, which is useful owing to its simple operation and enables point-of-care testing. Through this method, Park et al., (2020) devised a finger-actuated microfluidic device for spheroid cultivation and analysis that facilitates programmed media exchange and media injection for further analysis
[10][29]. Different sizes of BT474 spheroids were generated after seven days of growing and further analyzed in a LIVE/DEAD assay, indicating its spheroid growth in a manipulated ECM-mimicking environment. The proposed microfluidic-based device can be widely applied in biomedical laboratories by combining it with automated machinery. For the formation of the MCTs, the manipulation of cell size uniformity and long-term cultivation is difficult to control. To overcome this limitation, thermoresponsive copolymers with a poly(N-isopropylacrylamide) (p(NIPA)) backbone were manufactured
[11][30]. In this study, small-size spheroids of human breast adenocarcinoma cells were generated up to 2000 cells per drop. This demonstrated the superior performance of an in vivo 3D cell culture system. Through an immunofluorescence assay with the LIVE/DEAD analysis, these spheroids exhibited suitable drug penetration, making it a proper model for future drug-screening platforms. Hanging-drop is a conventional technology and has some limitations: (1) drops may fall off by mistake and (2) large amount of cells cannot be contained in one drop
[12][53].
Figure 12. (
A) Designing 3D-printed hanging-drop derivatives to investigate multicellular tumor spheroids. The device was printed for cancer spheroid formation on a 96/384 culture plate. Different types of assays are performed: drug screening, 3D metastasis, spheroid transendothelial migration, and spheroids merge/interactions. (
B) Characterization of different cell lines cultured over two days with varying ratios. Reprinted with permission from
[9][28]. Copyright 2019, Springer Nature.