You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Oscar Herrera-Calderon.
Quinoa (Chenopodium quinoa Willd.) is a pseudocereal belonging to the Amaranthaceae family that is native to the Andean region in South America. Peru is the leading quinoa-exporting country, exporting quinoa with a value of $98.5 million dollars, followed by Bolivia, the Netherlands, the United States, Spain, Germany, Canada, France, Ecuador, and Belgium.
- Amaranthaceae
- free radical
- flavonoids
- phenols
- amino acids
1. Introduction
Quinoa seeds are known to have a high protein content ranging from 11% to 19%. The seeds are a source of amino acids (isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, histidine, cysteine, tyrosine, glycine, arginine, proline, serine, glutamine, alanine, and aspartic acid), carbohydrates (49% to 68% dry weight), fat (2% to 9.5%), vitamins (thiamine, riboflavin, folic acid, and niacin), and minerals such as iron, zinc, magnesium, and copper (2.4% to 4.8%) [3][1]. Additionally, some phytochemical constituents such as saponins, phenolic compounds (ferulic, sinapinic and gallic acids, kaempferol, isorhamnetin, and rutin) [4][2], and peptides with therapeutic activity have been determined, making this crop very attractive for a wide range of food products [5][3]. Quinoa has been traditionally used in tortillas, pasta, flour, cookies, bread, and soups, among others, and is considered to be a gluten-free superfood and a source of fiber dietary [6][4]. Thus, quinoa is considered to be an acceptable food worldwide and is highly recommended for vegetarians.
On the other hand, sprouts are obtained by germinating the seeds and provide multiple nutritional and therapeutic benefits to those who consume them in different ways, due to the increase in the availability of nutrients such as fatty acids and carbohydrates, as well as polyphenols and flavonoids, during the germination process, which improves their antioxidant capacity [7][5]. These changes are due to a multitude of biochemical processes, which generate alterations in the composition of primary and secondary metabolites, producing an intrinsic change in the phenolic compounds and antioxidant activity [8][6]. Sprouts can improve the nutritional quality of a grain by eliminating or inactivating some antinutritional factors and increasing the digestibility of proteins and starches [9][7]. During germination, the original composition of the seed changes: the nitrogen-containing proteins move towards smaller protein fractions, oligopeptides, and free amino acids (some increase; others decrease or are not altered). Consequently, the changes increase the biological protein value of the sprouts, and digestibility is higher than in seeds [10][8].
Studies have reported that quinoa sprouts have high levels of amino acids, peptides, vitamins, and minerals but also include antinutritional components such as tannin, lectin, trypsin inhibitor, and galactoside, although at lower values than in non-germinated seeds [11][9]. The main enzyme involved in the early phase during the sprouting of quinoa seeds seems to be α-amylase, which leads to the generation of new compounds [12][10]. Some biological studies in quinoa sprouts have reported hepatoprotective, antioxidant [13][11], and anti-α-amylase effects in vitro [14][12], and hypoglycemic effects in diabetic rats [15][13].
2. Germination Process
Sprouts were obtained in a time of 72 h, and measured between 1.7 and 2.3 cm in length for all varieties. However, the red variety achieved the greatest length among all varieties (2.1–2.3 cm). The other varieties had lengths as follows: White Junín Ayacucho, 1.7–1.9 cm; T-256, 1.8–1.9 cm; Pasankalla, 1.7–1.8 cm; Suano Puno, 1.7–1.9 cm; T-38, 1.8–2.0 cm; Yellow Sacaca, 1.9–2.0 cm; T-45, 1.7–1.9 cm; Santa Ana, 1.7–1.8 cm; T-61 Pomata, 1.8–1.9 cm; CQA-048, 1.8–2.0 cm; Black Collana, 1.7–1.9 cm; T-72 Huancayo, 1.8–1.9 cm; CQA-043, 1.8–1.9 cm; Salcedo, 1.8–2.0 cm; Ayacucho Compuesto, 1.7–1.9 cm; White Choclito, 1.7–1.9 cm; Yellow Maranganí, 1.9–2.1 cm; Black Coito, 1.7–1.9 cm; and Black, 1.8–2.0 cm. Figure 1 shows the 20 varieties of quinoa germinated under standard laboratory conditions of temperature, humidity, and time.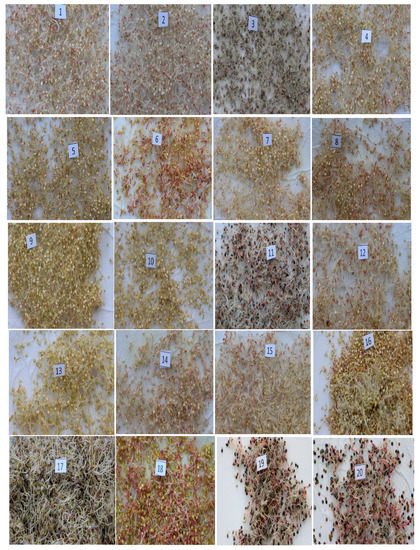
Figure 1. Twenty varieties of quinoa sprouts. (1), White Junín Ayacucho; (2), T-256; (3), Pasankalla; (4), Suano Puno; (5), T-38; (6), Yellow Sacaca; (7), T-45; (8), Santa Ana; (9), T-61 Pomata; (10), CQA-048; (11), Black Collana; (12), T-72 Huancayo; (13), CQA-043; (14), Salcedo; (15), Ayacucho Compuesto; (16), White Choclito; (17), Red; (18), Yellow Maranganí; (19), Black Coito; (20), Black.
3. Total Phenolic Content
The TPC of sprouts was found to range from 19.15 ± 1.54 to 31.28 ± 0.42 mg GAE/g of methanolic extract, being highest in the Pasankalla variety, CQA-048, Black Collana, and Black Coito. On the other hand, in quinoa seed extracts, the variation was from 11.72 ± 0.32 to 28.32 ± 0.49, being greater in the Pasankalla, Black Collana, and Black Coito varieties (Table 1). There was a significant difference between sprout and seed extracts for TPC, (paired sample t-test; p < 0.05), with TPC being higher in sprout extracts than in seed extracts, with an average of 24.57 ± 3.49 mg GAE/g in sprout extracts and 20.12 ± 4.37 mg GAE/g in seed extracts.
Table 1. Total phenolic content (TPC) and total flavonoids (TF) in the sprouts and seeds of 20 varieties of quinoa.
| Variety | TPC mg EAG/g ME |
TF mg EQ/g ME |
||
|---|---|---|---|---|
| Quinoa Sprouts Mean ± SD |
Quinoa Seeds Mean ± SD |
Quinoa Sprouts Mean ± SD |
Quinoa Seeds Mean ± SD |
|
|
23.32 ± 1.63 | 20.95 ± 0.79 | 11.52 ± 0.26 | 8.77 ± 0.26 * |
|
24.78 ± 0.21 | 13.82 ± 1.04 * | 11.23 ± 0.19 | 10.23 ± 0.95 |
|
31.28 ± 0.42 | 28.32 ± 0.49 * | 13.48 ± 0.38 | 11.52 ± 0.92 * |
|
19.62 ± 0.42 | 17.25 ± 0.66 * | 8.60 ± 0.48 | 8.56 ± 0.38 |
|
21.05 ± 0.40 | 21.75 ± 1.25 | 10.06 ± 0.57 | 9.81 ± 0.25 |
|
24.22 ± 0.31 | 23.58 ± 0.61 | 11.19 ± 0.38 | 8.23 ± 0.29 * |
|
21.02 ± 0.15 | 19.38 ± 2.06 | 11.06 ± 0.21 | 8.39 ± 0.38 * |
|
23.02 ± 0.74 | 18.23 ± 1.01 * | 9.94 ± 0.63 | 7.06 ± 0.33 * |
|
21.12 ± 1.50 | 15.55 ± 0.20 * | 10.94 ± 0.33 | 8.73 ± 0.31 * |
|
28.82 ± 0.67 | 21.32 ± 0.72 * | 7.44 ± 0.50 | 6.23 ± 0.26 * |
|
28.58 ± 1.21 | 26.98 ± 0.25 * | 13.44 ± 0.58 | 8.73 ± 0.14 * |
|
19.15 ± 1.54 | 18.58 ± 0.65 | 12.35 ± 0.48 | 9.81 ± 0.45 * |
|
26.05 ± 0.17 | 11.72 ± 0.32 * | 12.15 ± 0.08 | 11.31 ± 0.50 |
|
20.98 ± 1.99 | 12.38 ± 0.61 * | 11.94 ± 0.13 | 9.81 ± 0.45 * |
|
28.05 ± 0.53 | 21.42 ± 1.17 * | 11.19 ± 0.25 | 10.98 ± 0.40 |
|
24.02 ± 0.78 | 20.78 ± 1.86 | 11.52 ± 0.31 | 9.90 ± 0.26 * |
|
26.05 ± 0.36 | 20.45 ± 0.44 * | 12.31 ± 0.50 | 10.52 ± 0.19 * |
|
27.98 ± 0.70 | 22.82 ± 1.12 * | 13.52 ± 0.44 | 10.98 ± 0.52 * |
|
28.18 ± 0.35 | 24.42 ± 0.75 * | 14.31 ± 0.50 | 9.94 ± 0.13 * |
|
24.12 ± 0.64 | 20.78 ± 0.35 * | 12.31 ± 0.45 | 9.73 ± 0.38 * |
| Total Average ± SD | 24.57 ± 3.49 | 20.12 ± 4.37 * | 11.52 ± 1.67 | 9.46 ± 1.40 * |
* p < 0.05 (paired sample t-test); mg GAE/g ME: mg equivalent to gallic acid per g of methanolic extract.; mg EQ/g ME: mg equivalent to quercetin per g of methanolic extract.
4. Total Flavonoids
In sprouts, the flavonoid content varied from 7.44 ± 0.50 to 14.31 ± 0.5 mg EQ/g of extract, being highest in the Black Coito, Yellow Maranganí, Pasankalla, and Black Collana varieties. In seed extract, the variation was from 6.23 ± 0.26 to 11.52 ± 0.92 EQ/g. The results showed a significant increase in the total flavonoids in sprouts compared with seed extracts, but in some varieties, the increase was not significant, such as in T-256, Suano Puno, T-38, CQA-043, and Ayacucho Compuesto (Table 1).
5. Discussion
Polyphenolic compounds are secondary metabolites present in plants, which are divided into flavonoids and non-flavonoids, the first being responsible for the antioxidant capacity, exerting this through various mechanisms such as transition metal chelators, free radical scavengers, and enzyme inhibitors [16][14]. The antioxidant properties of secondary metabolites are related to vasodilatory, lipid-lowering, antiaging, and anti-inflammatory, modulating apoptosis processes in the vascular endothelium, but these molecules could also be influenced by factors such as the number and position of the phenolic hydroxyl groups, steric effects, and molecular properties [17][15]. In current results, the content of total phenols and flavonoids found in quinoa sprouts presented differences in each variety analyzed, being influenced by the type of seed, the cultivation site, maturity, storage, and germination conditions, as the flavonoids play an important role in pigmentation [18][16]. It is known that the phenolic compounds present in plants are formed during their development and under stress conditions; these include simple phenols, phenolic acids, coumarins, flavonoids, stilbenes, hydrolysable and condensed tannins, lignans, and lignins [19][17]. Additionally, these polyphenols could be altered during the germination process, increasing their content and the antioxidant capacity [20][18]. In current study, the variation in TPC and TF differed from the studies of Valencia et al., in which the TPC varied from 0.783 to 3437 mg GAE/g in quinoa seeds [21][19], and that of Carciochi et al. [22][20], with values of TPC of 39.3 ± 0.9 mg GAE/100 g and TF of 11.06 mg of quercetin/100 g in sprouts. These were higher in current study due to the type of solvent used in the maceration process. In the same way, when the antioxidant activity of the content of polyphenols and flavonoids was evaluated in the red and yellow varieties of quinoa, there was a significant increase after 9 days of germination. In a similar study, the antioxidant capacity in germinated seeds was greater compared with seeds of C. quinoa, increasing up to twofold, similar to the increase in phenolic compounds and antioxidant capacity observed after 72 h of germination [13][11]. In current study, a wide range of values were observed for phenolic compounds and flavonoids, as well as for the antioxidant activity in each variety of quinoa studied, which can be explained by the characteristics of each seed, variation in the availability of nutrients, and activation of the antioxidant machinery during germination. Several studies have shown nutritional improvements in quinoa sprouts, such as in crude quinoa flour (CQF) and germinated quinoa flour (GQF), where the CQF/GQF ratio increased the nutritional quality of pasta. Chemical analysis indicated an increase in the proportion of proteins by 37% and a decrease in phytic acid by 77%, which means that the germination process is an effective method to minimize phytic acid content in seeds. Pasta with a high CQF/GQF ratio had an increased content of Ca, K, Fe, Mn, Mg, P, and Zn, and thus using GQF is recommended in the production of bread, cakes, and cookies to take advantage of their nutritional properties, which provide a high content of proteins, minerals, TPC, and amino acids, and a low amount of phytic acid [23][21]. During germination, quinoa seeds undergo relevant physical and chemical changes; the maximum intensity of macromolecular modification occurs at 48 h. The germinated material contains micronutrients with improved bioavailability. This has a great impact on quinoa, as it improves the technological properties of quinoa, as well as some of its nutritional characteristics, enhancing the use of quinoa sprout flour as an ingredient in food formulation [12][10]. The germination process of quinoa seeds is an effective technique to enhance the content of total phenols and total flavonoids and to improve the antioxidant capacity, as was demonstrated in quinoa (C. quinoa) and kiwicha (Amaranthus caudatus) [24][22], where the sprouts had enhanced content of coumaric acid and kaempferol tri-glycoside in quinoa and caffeoylquinic acid in kiwicha. Additionally, a significant increase was observed in the phenolic content and the antioxidant capacity through malting quinoa sprouts [25][23] and Amaranthus caudatus sprouts [26][24].References
- Angeli, V.; Silva, P.M.; Massuela, D.C.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium Quinoa Willd.): An Overview of the Potentials of the “Golden Grain” and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods 2020, 9, 216.
- Gawlik-Dziki, U.; Świeca, M.; Sułkowski, M.; Dziki, D.; Baraniak, B.; Czyz, J. Antioxidant and Anticancer Activities of Chenopodium Quinoa Leaves Extracts—In Vitro Study. Food Chem. Toxicol. 2013, 57, 154–160.
- Hinojosa, L.; Leguizamo, A.; Carpio, C.; Muñoz, D.; Mestanza, C.; Ochoa, J.; Castillo, C.; Murillo, A.; Villacréz, E.; Monar, C.; et al. Quinoa in Ecuador: Recent Advances under Global Expansion. Plants 2021, 10, 298.
- Kuktaite, R.; Repo-Carrasco-Valencia, R.; de Mendoza, C.C.; Plivelic, T.S.; Hall, S.; Johansson, E. Innovatively Processed Quinoa (Chenopodium Quinoa Willd.) Food: Chemistry, Structure and End-Use Characteristics. J. Sci. Food Agric. 2021.
- Jang, H.W.; Moon, J.-K.; Shibamoto, T. Analysis and Antioxidant Activity of Extracts from Broccoli (Brassica Oleracea L.) Sprouts. J. Agric. Food Chem. 2015, 63, 1169–1174.
- Quassinti, L.; Gianfranceschi, G.; Lupidi, G.; Miano, A.; Bramucci, M. Antioxidant and Pro-Oxidant Activities of Savoy Cabbage (Brassica Oleracea L. Var. Sabauda) Sprout Extracts. J. Food Biochem. 2016, 40, 542–549.
- Niroula, A.; Khatri, S.; Khadka, D.; Timilsina, R. Total Phenolic Contents and Antioxidant Activity Profile of Selected Cereal Sprouts and Grasses. Int. J. Food Prop. 2019, 22, 427–437.
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421.
- Le, L.; Gong, X.; An, Q.; Xiang, D.; Zou, L.; Peng, L.; Wu, X.; Tan, M.; Nie, Z.; Wu, Q.; et al. Quinoa Sprouts as Potential Vegetable Source: Nutrient Composition and Functional Contents of Different Quinoa Sprout Varieties. Food Chem. 2021, 357, 129752.
- Suárez-Estrella, D.; Bresciani, A.; Iametti, S.; Marengo, M.; Pagani, M.A.; Marti, A. Effect of Sprouting on Proteins and Starch in Quinoa (Chenopodium Quinoa Willd.). Plant Foods Hum. Nutr. 2020, 75, 635–641.
- Al-Qabba, M.M.; El-Mowafy, M.A.; Althwab, S.A.; Alfheeaid, H.A.; Aljutaily, T.; Barakat, H. Phenolic Profile, Antioxidant Activity, and Ameliorating Efficacy of Chenopodium Quinoa Sprouts against CCl4-Induced Oxidative Stress in Rats. Nutrients 2020, 12, 2904.
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, Total Polyphenols and Antioxidant Activity in Amaranth and Quinoa Seeds and Sprouts during Their Growth. Food Chem. 2009, 115, 994–998.
- De Lopes, C.O.; de Barcelos, M.F.P.; de Vieira, C.N.G.; de Abreu, W.C.; Ferreira, E.B.; Pereira, R.C.; Angelis-Pereira, M.C. de Effects of Sprouted and Fermented Quinoa (Chenopodium Quinoa) on Glycemic Index of Diet and Biochemical Parameters of Blood of Wistar Rats Fed High Carbohydrate Diet. J. Food Sci. Technol. 2019, 56, 40.
- Hunyadi, A. The Mechanism(s) of Action of Antioxidants: From Scavenging Reactive Oxygen/Nitrogen Species to Redox Signaling and the Generation of Bioactive Secondary Metabolites. Med. Res. Rev. 2019, 39, 2505–2533.
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013.
- Pitzschke, A.; Fraundorfer, A.; Guggemos, M.; Fuchs, N. Antioxidative Responses during Germination in Quinoa Grown in Vitamin B-Rich Medium. Food Sci. Nutr. 2015, 3, 242–251.
- Lim, J.G.; Park, H.; Yoon, K.S. Analysis of Saponin Composition and Comparison of the Antioxidant Activity of Various Parts of the Quinoa Plant (Chenopodium Quinoa Willd.). Food Sci. Nutr. 2020, 8, 694.
- Zhang, Q.; Xing, B.; Sun, M.; Zhou, B.; Ren, G.; Qin, P. Changes in Bio-Accessibility, Polyphenol Profile and Antioxidants of Quinoa and Djulis Sprouts during in Vitro Simulated Gastrointestinal Digestion. Food Sci. Nutr. 2020, 8, 4232–4241.
- Valencia, Z.; Cámara, F.; Ccapa, K.; Catacora, P.; Quispe, F. Compuestos bioactivos y actividad antioxidante de semillas de quinua peruana (Chenopodium Quinoa W.). Rev. Soc. Química Perú 2017, 83, 16–29.
- Carciochi, R.A.; Manrique, G.; Dimitrov, K. Changes in Phenolic Composition and Antioxidant Activity during Germination of Quinoa Seeds (Chenopodium Quinoa Willd.). Int. Food Res. J. 2014, 21, 767–773.
- Demir, B.; Bilgiçli, N. Changes in Chemical and Anti-Nutritional Properties of Pasta Enriched with Raw and Germinated Quinoa (Chenopodium Quinoa Willd.) Flours. J. Food Sci. Technol. 2020, 57, 3884–3892.
- Pilco-Quesada, S.; Tian, Y.; Yang, B.; Repo-Carrasco-Valencia, R.; Suomela, J.P. Effects of Germination and Kilning on the Phenolic Compounds and Nutritional Properties of Quinoa (Chenopodium Quinoa) and Kiwicha (Amaranthus Caudatus). J. Cereal Sci. 2020, 94, 102996.
- Bhinder, S.; Kumari, S.; Singh, B.; Kaur, A.; Singh, N. Impact of Germination on Phenolic Composition, Antioxidant Properties, Antinutritional Factors, Mineral Content and Maillard Reaction Products of Malted Quinoa Flour. Food Chem. 2021, 346, 128915.
- Aguilar-Felices, E.J.; Romero-Viacava, M.; Enciso-Roca, E.; Herrera-Calderon, O.; Común-Ventura, P.; Yuli-Posadas, R.Á.; Chacaltana-Ramos, L.; Pari-Olarte, B. Antioxidant Activity of the Germinated Seed of Four Varieties of Amaranthus caudatus L. From Peru. Pharmacogn. J. 2019, 11.
More
