Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Uday Jha.
Chickpea (Cicer arietinum L.), the third most important grain legume in the world, is an important source of protein to human and animals in Asia and Africa. Consequently, major chickpea growing areas lie in these two continents; however, it is also cultivated in the USA, Canada, and Australia primarily for export to Asian and African countries. Chickpea evolved in the warm climates of the Mediterranean region and is thus sensitive to low temperatures.
- chilling
- legumes
- acclimatization
- stress
1. Introduction
Chickpea (Cicer arietinum L.), the third most important grain legume in the world, is an important source of protein to human and animals in Asia and Africa. Consequently, major chickpea growing areas lie in these two continents; however, it is also cultivated in the USA, Canada, and Australia primarily for export to Asian and African countries. Chickpea evolved in the warm climates of the Mediterranean region and is thus sensitive to low temperatures [1,2,3][1][2][3]. Chickpea experiences stressful low temperatures either during vegetative or reproductive growth, depending on the cultivation region [2,4,5,6][2][4][5][6]. In northern India and southern Australia, chickpea experiences low temperatures (<20/10 °C) during reproductive growth wherein cold stress damages leaves and flowers, decreases pollen and ovule fertility, impairs fertilization and alters the transcription in anthers and leaves [4], leading to flower and pod abortion and reducing the yield potential [4,6,7,8,9,10][4][6][7][8][9][10]. The threshold temperature for chickpea is 21 °C and temperatures below are stressful to chickpea; consequently, many production regions in the world are susceptible to cold stress [2,10][2][10].
Cold-stress-induced aberrations in crops at various organizational levels, including reduced vegetative and reproductive growth, delayed phenology, enhanced leaf chlorosis and necrosis, changes in leaf hydration status, flower abnormalities, and damage to reproductive structures and yield including chickpea are well understood [1,2,4,11][1][2][4][11]. Cold stress results in fewer numbers of pods and seeds per pod leading to lower yield [6]. In cold-sensitive chickpea genotypes, cold stress at all anther development stages i.e., micro- or mega-sporogenesis, gametogenesis and at mature pollen stage results in flower abortion [2]. The flower abortion is caused either by disruption of gametogenesis or abnormal pollen/ovule development that leads to sterility [2]. Younger flowers are relatively more sensitive to cold stress compared to old flowers as younger flowers do not have developed pollen grains whereas older flowers have developed pollen grains and results in sterility [2]. In older flowers, cold stress also decreases the ability of the pollen grains to germinate and retards pollen tube growth leading to failure or lack of fertilization resulting in poor seed set and fewer seeds per pod [2,4][2][4].
Despite significant advancements in our understanding of cold stress responses of chickpea, the metabolic and molecular mechanisms affecting cold sensitivity, especially in flowers, are relatively poorly understood [4,9,10][4][9][10]. At the cellular level, cold stress induces damage to membranes, increases production of reactive oxygen species (ROS), denatures enzymes and proteins and causes hormonal imbalance [12]. Our recent study [4] focused on the impact of cold stress on metabolites and enzymatic antioxidants as well as expression of genes of these pathways in anthers of cold-sensitive and cold-tolerant genotypes. While starch and proline were decreased in the cold-sensitive genotypes, there was no change in the cold-tolerant genotype. This decrease in sensitive genotype resulted from down-regulation of sucrose and proline transporter genes whereas there was up-regulation of these genes in cold-tolerant genotype [4]. It was shown that pollen viability of cold-tolerant genotypes was linked to maintenance of starch, reducing sugars and proline levels [4]. Additional studies are, however, needed to elucidate complete mechanisms associated with cold-induced flower abortion.
Plants, even cold-sensitive ones, also possess the ability to acquire cold tolerance. Cold tolerance acquisition takes place when plants are exposed to gradually decreasing low non-freezing temperatures, a process known as cold acclimation [13]. In general, acclimated plants may have greater cold tolerance compared to plants those are not acclimated [14,15,16][14][15][16]. Cold acclimation has been reported in several crops such as oilseed rape (Brassica napus; [17]), barley (Hordeum vulgare; [18]), and Arabidopsis thaliana [19]. In oilseed rape, maximum cold tolerance was achieved by exposure to 3 d of acclimation in spring cultivars and between 6 and 9 d in the winter cultivars, and cold tolerance decreased with prolonged acclimation duration [17]. At physiological level, the cold acclimation in barley led to significant changes in tissue water content, carbohydrate content and resulted in improved tillers and growth compared to non-acclimated plants [18]. Cold acclimation also modified the photosynthetic machinery and enabled plants to survive under severe cold temperatures through manipulation of chlorophyll a fluorescence [19]. Though information is not available for chickpea, in other crops, cold acclimation encompasses several mechanisms involving membrane changes [14], osmoprotectant accumulation (e.g., carbohydrates, proline, glycine betaine), antioxidant up-regulation [13,15][13][15] coupled with changes in expression of genes of these pathways [16]. These modifications during cold acclimation prepare cells to tolerate subsequent stressful low temperatures. It appears that the differential ability of the crops or their genotypes to tolerate cold stress depends on the types of physiological or biochemical changes during the process of cold acclimation.
2. Stress Injury to Leaves
2.1. Membrane Damage
Cold stress increased membrane damage (as electrolyte leakage; EL) in all four genotypes, more so in cold-sensitive genotypes. Cold-stressed tolerant genotypes had 18.4–20.5% EL (control: 10.5–12.5%), while cold-stressed sensitive genotypes had 26.3–28.3% EL (control: 10.1–13.4%; Figure 21A). Cold acclimation significantly reduced membrane damage in all genotypes, which decreased to 14.5–16.4% in tolerant genotypes and 20.6–21.3% in sensitive genotypes.
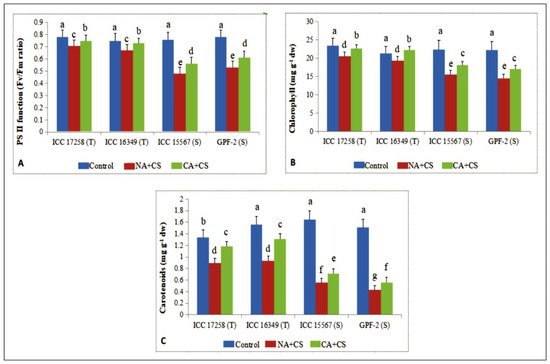

Figure 21. Membrane damage as electrolyte leakage (EL; (A)), relative leaf water content (RLWC; (B)) and stomatal conductance (gS; (C)) in leaves of control, non-acclimated, cold stressed; NA + CS) and cold-acclimated, cold stressed (CA + CS) plants of tolerant (T) and sensitive (S) genotypes. Small vertical bars represent standard errors (Mean ± S.E; n = 3). Different small letters on vertical bars indicate significant differences from each other (p < 0.05; Tukey’s test). Least significant difference (LSD) for interaction (p < 0.05): (genotypes × treatments) EL (2.4), RLWC (3.1) and gS (18.5).
3. Reproductive Traits
For reproductive parameters of male or female, cold-tolerant genotypes responded better to cold acclimation than cold-sensitive genotypes. The general effects of cold stress and cold acclimation on flowers, anthers and pollen grains are shown in Figure 42.
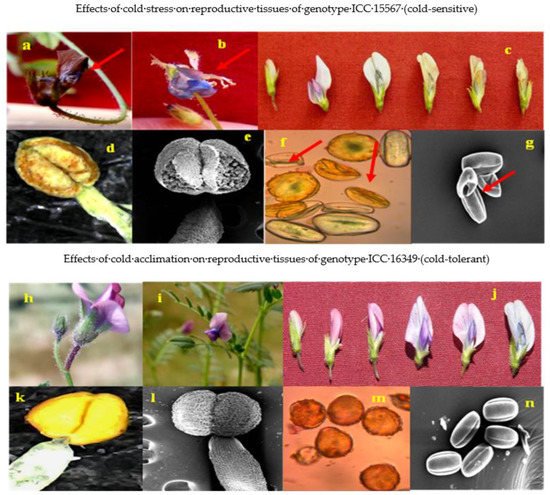

Figure 42. Images showing the effect of cold stress (above) and cold acclimation (below) on chickpea genotypes (reproductive phase). Cold stress effects from genotype ICC 15567 (cold-sensitive): aborted flower (a), flower with exposed anthers (b), developmental changes leading to abortion of flower (c), damaged anthers (d,e), and distorted and shriveled pollen grains (f,g). Cold acclimation effects from genotype ICC 16349 (cold-tolerant): flowers (h,i), developmental changes in cold-acclimated flowers (j), cold-acclimated anthers (k,l), and cold-acclimated viable pollen grains (m,n).
4. Discussion
As a winter season crop in several parts of the world, chickpea suffers from cold-stress-induced damage to vegetative and reproductive tissues. Studies have reported beneficial effects of cold acclimation for chickpea during the seedling or early vegetative phase [9[9][20][21],21,43], there are no reports investigating the impact of cold acclimation during the reproductive stage. In the present study, cold acclimation improved leaf, anther, and ovule function under cold stress, relative to non-acclimated plants, suggesting that cold acclimation is advantageous for vegetative and reproductive organs, improving plant growth, reproduction, and yield. Our study also showed that cold acclimation improved the response of vegetative tissues (leaves) to cold stress by reducing cold-induced damage and improving cellular function, such as membrane damage or relative leaf water content, stomatal conductance, PSII function, or leaf chlorophyll and carotenoid concentrations.
Cold acclimation can improve hardiness to cold stress [17] through various mechanisms. Cold acclimation can reduce membrane damage by increasing the ratio of unsaturated to saturated fatty acids, as reported in 20-day old chickpea seedlings [20][22]. We observed improved leaf water status in cold-acclimated chickpea plants, as reported in barley [18], and could be due to better root hydraulic conductivity and osmolyte accumulation [44][23]. The observed reduction in chlorophyll loss of cold-acclimated chickpea plants might have resulted from augmented leaf water status and reduced oxidative damage [9]. The reduction in chlorophyll and PSII function agrees with previous studies on cold-acclimated chickpea seedlings [43][21] and Arabidopsis thaliana (accession C24) [19] exposed to cold stress. Carotenoids are vital for maintaining the leaf redox status, protecting them from photoinhibition under cold stress [45][24], in our study, cold acclimation increased leaf carotenoid concentrations in cold-stressed chickpea, which might have protected the leaves from photoinhibition by adjusting the redox status and keeping the leaves photosynthetically active.
In non-acclimated chickpea plants exposed to cold stress, the marked reductions in growth, pod set, yield-related traits (pod and seed weights), and reproductive function could be associated with increased membrane damage and decreased water status, stomatal conductance, chlorophyll concentration, and PSII function in leaves. In chickpea, low temperature stress increased membrane damage [5] and decreased leaf hydration status and stomatal conductance could be due to reduced root hydraulic conductivity [46,47][25][26], chlorophyll [48][27], chlorophyll fluorescence [43][21], pollen function, stigmatic and ovular activity [4,8[4][8][28],49], and pod set and yield traits [7,10][7][10] Cold-stress-induced membrane disruption results from altered lipid–protein interactions [50][29] or lipid peroxidation [51][30], chlorophyll loss in cold-stressed plants, as observed in our study, might be due to inhibited chlorophyll synthesis or increased chlorophyll degradation [52][31] or photooxidation-induced disorganization of chloroplasts [53][32], which consequently decreases chlorophyll fluorescence [43][21]. Leaf damage due to cold stress can disrupt photosynthetic function and sucrose synthesis and transport to developing floral organs, causing impaired reproductive function and reduced yields [54][33].
Unlike leaf tissues, the response of reproductive organs to cold acclimation in cold-tolerant and cold-sensitive chickpea genotypes differed. The zero pod set and zero yield in cold-acclimated cold-sensitive genotypes under cold stress indicates the lack of a cold acclimatization response. In contrast, cold-tolerant genotypes had a cold acclimation response (increased pod and seed set relative to non-acclimated plants). Interestingly, our findings and those of [9] indicate that vegetative and reproductive tissues of cold-sensitive chickpea genotypes differ in their response to cold acclimation. Indeed, reproductive organs (anthers and ovules) had significantly more tissue damage and less cellular viability in cold-sensitive genotypes than cold-tolerant genotypes; moreover, these organs were less responsive to cold acclimation in sensitive genotypes. Thus, the differential response to cold acclimation might lie in the tissue sensitivity of floral organs in cold-sensitive and cold-tolerant genotypes, as indicated by various traits related to tissue damage, but this aspect needs further study.
In chickpea, cold stress reduces pollen viability, pollen load on stigma, stigma receptivity, and ovule viability [4,49][4][28]. Chickpea plants fail to set pods at temperatures <20/10 °C due to various abnormalities related to developmental and functional factors [1,4,8,49][1][4][8][28]. In cold-tolerant chickpea genotypes, cold acclimation reduced the adverse effect of cold stress, increasing yield. Little or no cold acclimation of reproductive organs in cold-sensitive genotypes might be due to poor expression of enzymatic and non-enzymatic antioxidants and reduced accumulation of cryoprotective molecules in reproductive organs. The cold-sensitive genotypes were unable to significantly reduce cold-stress-induced oxidative stress markers, such as MDA and H2O2, in both male and female reproductive organs following acclimation. Consequently, these genotypes failed to detoxify ROS following the production of those by lower temperatures, impairing male and female gamete function and causing flower/pod abortion.
The role of ROS is well documented for sensitivity to abiotic stresses [55][34]. In chickpea, cold stress affects male and female gamete function, resulting in poor pollen germination, viability, stigmatic receptivity, and ovule viability [4,9,49,56][4][9][28][35]. The current study showed that cold stress caused tissue damage in anthers and ovules and reduced their cellular viability. The manifold increase in oxidative stress in anthers and ovules under cold stress points to its role in tissue damage and cell viability in these organs. Therefore, it cannot be ruled out that oxidative-stress-induced tissue damage disrupts developmental and functional aspects of anthers and ovules. In rice anthers, ROS accumulation has been reported under drought [57][36], and heat stress [58][37]. In cytoplasmic male sterile (CMS) rice material, the CMS line (sterile anthers) had significantly higher ROS concentrations in anthers than the corresponding maintainer line (fertile anthers) [59,60][38][39].
The cold acclimation response of cold-tolerant chickpea genotypes could be attributed to a substantial reduction in MDA and H2O2levels in anthers and ovules and increased accumulation of antioxidants (enzymatic and non-enzymatic). An increase in enzymatic and non-enzymatic antioxidant levels reduced the oxidative species generated under cold stress, thus reducing the oxidative stress in anthers and ovules to levels too low to cause considerable damage to these organs. Thus, reduced oxidative damage to these organs improved anther and ovule performance under cold stress in cold-acclimated plants, relative to non-acclimated plants in the cold-tolerant genotypes. Decreased production of oxidative species and increased production of antioxidants leads to cold tolerance in crops such as rice (Oryza sativa L.) [61][40] and Brassica sp. [62][41]. Cold acclimation improved the antioxidant capacity of barley [63][42] and chickpea [64][43] leaves.
Numerous studies have demonstrated that the antioxidant enzyme system in plants can protect against ROS, but little is known about antioxidant enzymes in developing anthers [4], or the interaction between cold-induced ROS concentrations in anthers and ovules of chickpea. In some crops, antioxidant enzymes reduce ROS-induced damage and are important components of plant tolerance to environmental stresses [65,66][44][45]. In the present study, the activities of SOD (causes dismutation of peroxides), CAT (detoxifies the hydrogen peroxide), APX (detoxifies hydrogen peroxide using ascorbate as a substrate), and GR (catalyzes the reduction of glutathione disulfide to the sulfhydryl form GSH) increased in anthers and ovules of non-acclimated cold-tolerant genotypes, indicating an inherent ability of these genotypes to reduce cold-induced oxidative stress. However, the reduction in pod numbers in cold-tolerant non-acclimated genotypes exposed to cold stress suggests that the decrease in oxidative damage in anthers and ovules was not significant. In contrast, cold-sensitive genotypes had much lower antioxidant levels in anthers and ovules than cold-tolerant genotypes, causing severe oxidative damage to these organs, manifested as inhibited reproductive function and lack of pod set. The considerably greater reduction in tissue damage (as EL and cellular viability) in anthers and ovules of cold-tolerant genotypes than cold-sensitive genotypes might be due to an improvement in unsaturation of lipids [67][46], and reduction in oxidative stress in acclimated plants. Like anthers and ovules, cold acclimation reduced the severity of oxidative stress in chickpea seedlings [21][20] and barley leaves [63][42]. Variations, however, have been reported in the activities and the type of antioxidants in cold-acclimated plants, which might depend on the experimental conditions and plant species used [63,68][42][47]. In the present study, components of the ascorbate–glutathione pathway were greatly expressed, compared to other antioxidative enzymes, suggesting their larger role in the cold acclimation potential of cold-tolerant genotypes.
Cryoprotective molecules can maintain reproductive function in plants. Following cold acclimation, the anthers and ovules of cold-sensitive genotypes accumulated lesser amounts of cryoprotective molecules, such as proline, GABA, trehalose, and sucrose, compared to cold-tolerant genotypes. Our previous study [8] on cold-stressed chickpea revealed an association between reduced carbohydrates in ovules and floral abortion. The cold acclimation of cold-tolerant genotypes can thus be attributed to the inherent ability of these genotypes to reduce oxidative stress and enhance antioxidant levels (enzymatic and non-enzymatic) and cryoprotective solutes in reproductive organs (anthers and ovules), improving reproductive function, e.g., pollen viability, pollen load on stigma, stigma receptivity and ovule viability, and subsequently number of pods and seeds.
Cold acclimation can enhance endogenous proline (Chrysanthemum sp.) [69][48], carbohydrates (safflower, Carthamus tincotorius) [70][49], and GABA (barley and wheat; [71][50] levels in plants. Cryoprotective solutes, such as amino acids (proline, GABA) and carbohydrates (sucrose, trehalose), play diverse roles in plant cells [72][51]. Moreover, its role as an osmolyte in osmotic adjustment, proline stabilizes membranes and proteins, scavenges free radicals, and buffers cellular redox potential under stress conditions [73][52]. The importance of proline in cold stress mitigation can be judged because it has been used as a biomarker of cold tolerance [74][53]. In cold-tolerant chickpea under cold stress, higher proline levels were attributed to increased expression of the gene responsible for proline transport, proline transporter 1 [4]. GABA is a non-protein amino acid—it has a signaling role with functions to protect from oxidative stress, maintain C and N mechanism, regulate pH in cytosol, and in osmoregulation [75][54] and cold tolerance [76][55]. Trehalose (α-D-glucopyranosyl- α-D-glucopyranoside) is a vital compatible sugar solute—it has a signaling role and stabilizes lipid membranes, dehydrated enzymes, and proteins during desiccation [77][56]. It has also been implicated in acquiring stress tolerance in plants, including cold stress [78][57]. Sucrose has been implicated in conferring cold tolerance [79][58] and can directly protect cell membranes by interacting with the phosphate in their lipid headgroups, thus decreasing membrane permeability [80][59]. Non-acclimated cold-tolerant chickpea genotypes had substantially higher levels of these solutes than non-acclimated cold-sensitive genotypes, suggesting their involvement in cold tolerance. However, their concentrations may have been inadequate to maintain reproductive competence. The depletion of proline, sucrose, and reducing sugars in flowers due to impaired mobilization and synthesis causes flower abortion due to decreased pollen viability and retarded pollen tube growth [9,56][9][35].
Sucrose, in addition to a cryoprotectant, might act as a source of carbon to developing anthers and ovules. Adequate carbohydrate supply is critical for anther function under cold stress [81][60] and sucrose is an important carbohydrate molecule required for proper anther function, especially under stress, e.g., in tomato (Solanum lycopersicum) [82][61] and chickpea [8]. In an earlier study, the expression of sucrose-synthesizing genes was compared in anthers of cold-stressed cold-tolerant and cold-sensitive chickpea genotypes [4]. Under cold stress, the anthers of cold-tolerant genotype, ICC 16349, had higher pollen viability than cold-sensitive, GPF2. Increased pollen viability in the cold-tolerant genotype was associated with up-regulation of sucrose-synthesizing genes, UDP glucose pyrophosphorylase, sucrose phosphate synthase2, and CWIN cell wall invertase [4].
PCA graphs of non-acclimated and cold-acclimated treatments of chickpea genotypes demonstrated strong correlation among reproductive, biochemical, anti-oxidative and yield traits. At the same time, cold-acclimated plants showed increased protective traits (CAR, Chl, CV, SC, PSII, CAT, SOD, APX, GR, ASC, proline) as compared to non-acclimated plants so that plants could achieve cold tolerance. Furthermore, cold-acclimated plants showed higher reproductive traits (PG, PV, SR, OV) than non-acclimated plants that may result in enhanced yield traits (pod set %, pod number plant–1, seed weight plant–1). In contrast, non-acclimated plants showed significant chilling injury traits (EL, MDA, H2O2) as compared to cold-acclimated plants. Moreover, there was a strongly positive correlation among various protective, reproductive and yield traits in cold-acclimated plants as compared to non-acclimated plants. Thus, cold-acclimated plants acquired substantial cold tolerance that leads to increased yield.
References
- Croser, J.S.; Clarke, H.J.; Siddique, K.H.M.; Khan, T.N. Low-temperature stress: Implications for chickpea (Cicer arietinum L.) improvement. Crit. Rev. Plant. Sci. 2003, 22, 185–219.
- Rani, A.; Devi, P.; Jha, U.C.; Sharma, K.D.; Siddique, K.H.; Nayyar, H. Developing climate-resilient chickpea involving physiological and molecular approaches with a focus on temperature and drought stresses. Front. Plant Sci. 2020, 10, 1759.
- Singh, K.B.; Malhotra, R.S.; Halila, M.H.; Knights, E.J.; Verma, M.M. Current status and future strategy in breeding chickpea for resistance to biotic and abiotic stresses. Euphytica 1993, 73, 137–149.
- Kiran, A.; Sharma, P.N.; Awasthi, R.; Nayyar, H.; Seth, R.; Chandel, S.S.; Sharma, K.D. Disruption of carbohydrate and proline metabolism in anthers under low temperature causes pollen sterility in chickpea. Environ. Exp. Bot. 2021, 188, 104500.
- Mir, A.H.; Bhat, M.A.; Dar, S.A.; Sofi, P.A.; Bhat, N.A.; Mir, R.R. Assessment of cold tolerance in chickpea (Cicer spp.) grown under cold/freezing weather conditions of North-Western Himalayas of Jammu and Kashmir, India. Physiol. Mol. Biol. Plants 2021, 27, 1105–1118.
- Srinivasan, A.; Johansen, C.; Saxena, N.P. Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L.): Characterization of stress and genetic variation in pod set. Field Crop. Res. 1998, 57, 181–193.
- Clarke, H.J.; Siddique, K.H.M. Response of chickpea genotypes to low temperature stress during reproductive development. Field Crop. Res. 2004, 90, 323–334.
- Nayyar, H.; Bains, T.; Kumar, S. Low temperature induced floral abortion in chickpea: Relationship to abscisic acid and cryoprotectants in reproductive organs. Environ. Exp. Bot. 2005, 53, 39–47.
- Nayyar, H.; Bains, T.S.; Kumar, S. Chilling stressed chickpea seedlings: Effect of cold acclimation, calcium and abscisic acid on cryoprotective solutes and oxidative damage. Environ. Exp. Bot. 2005, 54, 275–285.
- Berger, J.D.; Kumar, S.; Nayyar, H.; Street, K.A.; Sandhu, J.S.; Henzell, J.M.; Clarke, H.C. Temperature-stratified screening of chickpea (Cicer arietinum L.) genetic resource collections reveals very limited reproductive chilling tolerance compared to its annual wild relatives. Field. Crop. Res. 2012, 126, 119–129.
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443.
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560.
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599.
- Orvar, B.L.; Sangwan, V.; Omann, F.; Dhindsa, R.S. Early steps in cold sensing by plant cells: The role of actin cytoskeleton and membrane fluidity. Plant J. 2000, 23, 785–794.
- Kang, H.M.; Saltveit, M.E. Activity of enzymatic antioxidant defense systems in chilled and heat shocked cucumber seedling radicles. Physiol. Plant. 2001, 113, 548–556.
- Pearce, R.S. Molecular analysis of acclimation to cold. Plant Growth Regul. 1999, 29, 47–76.
- Rife, C.L.; Zeinali, H. Cold tolerance in oilseed rape over varying acclimation durations. Crop Sci. 2003, 43, 96–100.
- Burchett, S.; Niven, S.; Fuller, M.P. The effect of cold-acclimation on the water relations and freezing tolerance of Hordeum vulgare L. Cryo. Letters 2006, 27, 295–303.
- Mishra, K.B.; Mishra, A.; Kubasek, J.; Urban, O.; Heyer, A.G. Low temperature induced modulation of photosynthetic induction in non-acclimated and cold-acclimated Arabidopsis thaliana: Chlorophyll a fluorescence and gas-exchange measurements. Photosyn. Res. 2019, 139, 123–143.
- Turan, O.; Ekmekçi, Y. Chilling tolerance of Cicer arietinum lines evaluated by photosystem II and antioxidant activities. Turk. J. Bot. 2014, 38, 499–510.
- Turan, O.; Ekmekçi, Y. Activities of photosystem II and antioxidant enzymes in chickpea (Cicer arietinum L.) cultivars exposed to chilling temperatures. Acta Physiol. Plant. 2011, 33, 67–78.
- Kazemi-Shahandashti, S.S.; Maali-Amiri, R.; Zeinali, H.; Khazaei, M.; Talei, A.; Ramezanpour, S.S. Effect of short-term cold stress on oxidative damage and transcript accumulation of defense-related genes in chickpea seedlings. J. Plant Physiol. 2014, 171, 1106–1116.
- Sarkar, P.; Bosneaga, E.; Auer, M. Plant cell walls throughout evolution: Towards a molecular understanding of their design principles. J. Exp. Bot. 2009, 60, 3615–3635.
- Frank, H.A.; Brudvig, G.W. Redox functions of carotenoids in photosynthesis. Biochemistry 2004, 43, 8607–8615.
- Aroca, R.; Irigoyen, J.J.; Sanchez-Diaz, M. Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiol. Plant. 2003, 117, 540–549.
- Lee, S.H.; Singh, A.P.; Chung, G.C.; Ahn, S.J.; Noh, E.K.; Steudle, E. Exposure of roots of cucumber (Cucumis sativus) to low temperature severely reduces root pressure, hydraulic conductivity and active transport of nutrients. Physiol. Plant. 2004, 120, 413–420.
- Amini, S.; Maali-Amiri, R.; Kazemi-Shahandashti, S.S.; Lopez-Gomez., M.; Sadeghzadeh, B.; Sobhani-Najafabadi, A.; Kari-man, K. Effect of cold stress on polyamine metabolism and antioxidant responses in chickpea. J. Plant Physiol. 2021, 258–259, 153387.
- Kiran, A.; Kumar, S.; Nayyar, H.; Sharma, K.D. Low temperature-induced aberrations in male and female reproductive organ development cause flower abortion in chickpea. Plant Cell Environ. 2019, 42, 2075–2089.
- Uemura, M.; Joseph, R.A.; Steponkus, P.L. Cold acclimation of Arabidopsis thaliana (effect on plasma membrane lipid composition and freeze-induced lesions). Plant Physiol. 1995, 109, 15–30.
- Chaki, T.; Hirata, N.; Yoshikawa, Y.; Tachibana, S.; Tokinaga, Y.; Yamakage, M. Lipid emulsion, but not propofol, induces skeletal muscle damage and lipid peroxidation. J. Anesth. 2019, 33, 628–635.
- Tewari, A.K.; Tripathy, B.C. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998, 117, 851–858.
- Camejo, D.; Jimenez, A.; Alarcon, J.J.; Torres, W.; Gomez, J.M.; Sevilla, F. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct. Plant Biol. 2006, 33, 177–187.
- Kaur, G.; Kumar, S.; Nayyar, H.; Upadhyaya, H.D. Cold stress injury during the pod-filling phase in chickpea (Cicer arietinum L.): Effects on quantitative and qualitative compo-nents of seeds. J. Agron. Crop Sci. 2008, 194, 457–464.
- Gechev, T.; Petrov, V. Reactive Oxygen Species and Abiotic Stress in Plants. Int. J. Mol. Sci. 2020, 21, 7433.
- Srinivasan, A.; Saxena, N.P.; Johansen, C. Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L.): Genetic variation in gamete development and function. Field Crop. Res. 1999, 60, 209–222.
- Fu, G.F.; Jian, S.O.N.G.; Xiong, J.; Li, Y.R.; Chen, H.Z.; Le, M.K.; Tao, L.X. Changes of oxidative stress and soluble sugar in anthers involve in rice pollen abortion under drought stress. Agric. Sci. China 2011, 10, 1016–1025.
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24.
- Li, S.; Wan, C.; Kong, J.; Zhang, Z.; Li, Y.; Zhu, Y. Programmed cell death during microgenesis in a Honglian CMS line of rice is correlated with oxidative stress in mitochondria. Funct. Plant Biol. 2004, 31, 369–376.
- Wan, Z.; Jing, B.; Tu, J.; Ma, C.; Shen, J.; Yi, B.; Fu, T. Genetic characterization of a new cytoplasmic male sterility system (hau) in Brassica juncea and its transfer to B. napus. Theor. Appl. Genet. 2008, 116, 355–362.
- Nanculao, G.D.; Herrera, M.L.; Carcamo, M.P.; Velasquez, V.B. Relative expression of genes related with cold tolerance in temperate rice at the seedling stage. Afr. J. Biotechnol. 2014, 13, 2506–2512.
- Soengas, P.; Rodriguez, V.M.; Velasco, P.; Cartea, M.E. Effect of temperature stress on antioxidant defenses in Brassica oleracea. ACS Omega 2018, 3, 5237–5243.
- Dai, K.; Peng, T.; Ke, D.; Wei, B. Photocatalytic hydrogen generation using a nanocomposite of multi-walled carbon nanotubes and TiO2 nanoparticles under visible light irradiation. Nanotechnology 2009, 20, 125603.
- Nazari, M.; Amiri, R.M.; Mehraban, F.H.; Khaneghah, H.Z. Change in antioxidant responses against oxidative damage in black chickpea following cold acclimation. Russian J. Plant Physiol. 2012, 59, 183–189.
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Role of tocopherol (vitamin E) in plants: Abiotic stress tolerance and beyond. In Emerging Technologies and Management of Crop Stress Tolerance; Academic Press: London, UK, 2014; pp. 267–289.
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69.
- Wodtke, E. Temperature adaptation of biological membranes. The effects of acclimation temperature on the unsaturation of the main neutral and charged phospholipids in mitochondrial membranes of the carp (Cyprinus carpio L.). Biochim. Biophys. Acta Biomembr. 1981, 640, 698–709.
- Pennycooke, J.C.; Cox, S.; Stushnoff, C. Relationship of cold acclimation, total phenolic content and antioxidant capacity with chilling tolerance in petunia (Petunia× hybrida). Environ. Exp. Bot. 2005, 53, 225–232.
- Chen, Y.; Jiang, J.; Chang, Q.; Gu, C.; Song, A.; Chen, S.; Chen, F. Cold acclimation induces freezing tolerance via antioxidative enzymes, proline metabolism and gene expression changes in two chrysanthemum species. Mol. Biol. Rep. 2014, 41, 815–822.
- Landry, E.J.; Fuchs, S.J.; Bradley, V.L.; Johnson, R.C. The effect of cold acclimation on the low molecular weight carbohydrate composition of safflower. Heliyon 2017, 3, e00402.
- Mazzucotell, E.; Belloni, S.; Marone, D.; De Leonardis, A.M.; Guerra, D.; Di Fonzo, N.; Cattivelli, L.; Mastrangelo, A.M. The E3 ubiquitin ligase gene family in plants: Regulation by degradation. Curr. Genom. 2006, 7, 509–522.
- Bhandari, K.; Sharma, K.D.; Rao, B.H.; Siddique, K.H.; Gaur, P.; Agrawal, S.K.; Nayyar, H. Temperature sensitivity of food legumes: A physiological insight. Acta Physiol. Plant. 2017, 39, 68.
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466.
- Vera-Hernandez, P.; Ortega Ramirez, M.A.; Martinez Nunez, M.; Ruiz-Rivas, M.; Rosas-Cárdenas, F.D.F. Proline as a probable biomarker of cold stress tolerance in sorghum (Sorghum bicolor). Mex. J. Biotechnol. 2018, 3, 77–86.
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115.
- Malekzadeh, P.; Khara, J.; Heydari, R. Alleviating effects of exogenous Gamma-aminobutyric acid on tomato seedling under chilling stress. Physiol. Mol. Biol. Plants 2014, 20, 133–137.
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. 2019 Trehalose: A key organic osmolyte effectively involved in plant abiotic stress tolerance. J. Plant Growth Regul. 2019, 38, 606–618.
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Do Choi, Y.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903.
- Tabaei-Aghdaei, S.R.; Pearce, R.S.; Harrison, P. Sugars regulate cold-induced gene expression and freezing-tolerance in barley cell cultures. J. Exp. Bot. 2003, 54, 1565–1575.
- Strauss, G.; Hauser, H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc. Natl. Acad. Sci. USA 1986, 83, 2422–2426.
- Parish, R.W.; Phan, H.A.; Iacuone, S.; Li, S.F. Tapetal development and abiotic stress: A centre of vulnerability. Funct. Plant Biol. 2012, 39, 553–559.
- Pressman, E.; Harel, D.; Zamski, E.; Shaked, R.; Althan, L.; Rosenfeld, K.; Firon, N. The effect of high temperatures on the expression and activity of sucrose-cleaving enzymes during tomato (Lycopersicon esculentum) anther development. J. Hort. Sci. Biotechnol. 2006, 81, 341–348.
More
