Cisplatin is a chemotherapy agent commonly used to treat a wide variety of cancers. Despite the potential for both severe acute and chronic side effects, it remains a preferred therapeutic option for many malignancies due to its potent anti-tumor activity. Common cisplatin-associated side-effects include acute kidney injury (AKI) and chronic kidney disease (CKD). These renal injuries may cause delays and potentially cessation of cisplatin therapy and have long-term effects on renal function reserve. Thus, developing mechanism-based interventional strategies that minimize cisplatin-associated kidney injury without reducing efficacy would be of great benefit. In addition to its action of cross-linking DNA, cisplatin has been shown to affect mitochondrial metabolism, resulting in mitochondrially derived reactive oxygen species (ROS). Increased ROS formation in renal proximal convoluted tubule cells is associated with cisplatin-induced AKI and CKD. We review the mechanisms by which cisplatin may induce AKI and CKD and discuss the potential of mitochondrial superoxide dismutase mimetics to prevent platinum-associated nephrotoxicity.
- superoxide dismutase
- mitochondria
- reactive oxygen species
- mitochondrial metabolism
- superoxide
The use of cisplatin as an anticancer agent was first published in 1969, describing its action against malignant murine sarcoma and leukemia [38]. Higby and Wallace investigated cisplatin in metastatic testicular cancer, wherein they reported seven cases of complete recovery and 13 cases of significant tumor regression in a 15-patient clinical study [39]. Einhorn and Donohue combined cisplatin with bleomycin and vinblastine for advanced testicular cancer [40]. This three-drug regimen had an initial 70% complete re-sponse rate and five-year survival rate of 64% [40]. Wiltshaw and colleagues reported similar outcomes for advanced ovarian cancer using cisplatin as a single agent in 82 ovarian cancer patients previously treated with conventional chemotherapy [41]. Ovarian cancer response rate was dose-dependent, ranging between 33% for a 30 mg/m
1. Cisplatin as a Treatment Modality for Cancer
2 dose and 52% for a 100 mg/m
2 dose [41]. On the basis of the success of these trials, cisplatin expanded to include additional malignancies such as cervical, lung, and head and neck cancers [34]. The outcomes were consistent with the testicular and ovarian cancer studies, wherein cisplatin was effective both as a single agent and in combination with other chemotherapeutic agents [42].
With cisplatin’s promising clinical trial success as an anti-cancer therapy, it became vital to understand this novel drug’s underlying mechanism of action. In 1970, Rosenberg and VanCamp proposed that cisplatin stimulated an immune response [43]. Later studies in mammalian cells and animals treated with cisplatin then revealed that the drug inhibits DNA synthesis and cell growth [44,45]. This discovery was made by tracing the incorporation of the radioactive DNA, RNA, and protein precursors 3H-thymidine, 3H-uridine, and 3H-L-leucine, respectively. Cisplatin hindered 3H-thymidine incorporation into DNA but not 3H-uridine or 3H-L-leucine incorporation both in vitro and in vivo [44,45]. It is now established that cisplatin binds to DNA purines at the N7 position and forms 1-, 2-, or 3-intrastrand crosslinks that terminate DNA replication and transcription and recruit high-mobility group box protein 1 (HMGB1), leading to the activation of pathways associated with DNA damage and apoptosis, such as p53 and MAPK [46].
Another noteworthy aspect of cisplatin’s history as an anti-cancer therapy is its radiation sensitizing activity. In 1978, Alvarez and colleagues reported that cisplatin sensitized TC.SV-40 cells against ionizing radiation in vitro [47]. As cisplatin showed efficacy as a chemotherapeutic agent in clinical trials, it was also tested in combination with radiotherapy. In 1981, 124 patients with advanced inoperable squamous cell carcinoma of the head and neck received cisplatin (100 mg/m
2) every three weeks concurrently with definitive radiotherapy (planned total dose ≥ 64.5 Gy) [48]. Patients in this trial had significantly improved clinical response rates that differed on the basis of tumor site and differentiation state. Patients with hypopharyngeal cancer responded 25% of the time, while patients with nasopharyngeal tumors responded 83% of the time. The response rate for poorly differentiated tumors was 89% compared to 67% and 59% for well-differentiated and moderately differentiated tumors, respectively [46]. However, severe toxicities associated with this treatment regimen included leukopenia (11%), nausea and vomiting (8%), stomatitis (31%), and nephrotoxicity (6%) [48].
Subsequent randomized clinical trials have shown concurrent cisplatin improves locoregional control, progression-free survival, and overall survival in non-small cell lung cancer [49], cervical cancer [50], and head and neck cancer [51–53] over radiation alone, induction chemotherapy, or radiation in combination with other agents. Rates of severe toxicities from concurrent cisplatin in these trials include leukopenia (11–42%), nausea and vomiting (8–28%), stomatitis (31–43%), anemia (17%), dermatitis (7%), neurologic toxicity (5%), and nephrotoxicity (4–8%) [48–53]. Any grade acute kidney injury incidence is as high as 34% with high dose cisplatin (100 mg/ q3 weeks) [54]. The risk of cisplatin-induced nephrotoxicity increases with cisplatin dose and duration of treatment [55]. For example, 34% of head and neck cancer patients treated with fractionated ionizing radiation (total dose of 60 to 70 Gy in 2 Gy fractions) and cisplatin therapy (100 mg/m
2 delivered every 21 days for 3 cycles) develop cisplatin-induced AKI [54]. A decline in renal function may necessitate cisplatin administration delays and dose reductions as patients cannot receive a planned dose of cisplatin [54]. Risk factors for developing nephrotoxicity following cisplatin exposure are related to the renal clearance of cisplatin. Patients prone to developing AKI following cisplatin treatment include those that have high peak plasma cisplatin concentrations (>400 ng/mL) [52], pre-existing kidney damage (creatinine > 1.5 mg/dL) [53], age ≥ 61 years, and a history of hypertension [56,57]. Survivors of childhood cancers treated with cisplatin (cumulative doses > 450 mg) develop long-term (decades) nephrotoxicity with reduced estimated glomerular filtration rates compared to childhood cancer survivors not treated with cisplatin (eGFR of 83 mL/min/1.73 m
2
2
2
2
2
2 for seven doses). Patients treated with cisplatin had an improved 5-year progression-free survival (78% vs. 67%) and reduced 5-year local regional failure (9.9% vs. 17%). There were no significant differences in xerostomia, fibrosis, muscle atrophy, and weight loss. On the basis of these data, the research found that radiation combined with cisplatin is superior to radiation combined with cetuximab for the definitive treatment of locally advanced oropharyngeal carcinoma [52].
Despite its treatment efficacy, cisplatin treatment is known to cause significant toxicities. A phase III intergroup trial in head and neck cancer patients comparing subjects that received radiation alone (70 Gy/35 fx), radiation and cisplatin (100 mg/m
2 on days 1, 22, and 43), or split course radiation was given with three cycles of 5-fluorouracil and cisplatin chemotherapy, identifying improved 3-year overall survival in patients treated with concurrent cisplatin and radiation . Relative to subjects receiving radiation alone, however, subjects treated with concurrent cisplatin and radiation had an increased risk for ≥ grade 3 nausea and vomiting (16% vs. 6%), leukopenia (42% vs. 1%), anemia (17% vs. 0%), and nephrotoxicity (8% vs. 1%).
A retrospective review of 821 adult cancer survivors treated with cisplatin who survived for at least 5 years demonstrated the following changes in renal function: patients who were CKD stage 1 pre-cisplatin treatment progressed to CKD stage 2 (48%) or CKD stage 3 (14%), while only 36% remained at CKD stage 1 [23].
A common clinical approach to prevent and reduce the severity of cisplatin-associated nephrotoxicity is pre-hydration with intravenous isotonic saline to increase diuresis [58]. Additional common clinical approaches include avoiding concomitant nephrotoxic drugs, reducing cisplatin dose [59], and substituting an alternative chemotherapy agent for cisplatin [60]. Examples of additional approaches that are less commonly utilized clinically include amifostine and theophyilline. Amifostine is approved by the FDA to reduce renal injury associated with multiple cisplatin administrations [61,62]. Amifostine is a thiol derivative that scavenges free radicals generated during radiation and chemotherapy [63]. Pre-clinical studies demonstrate that amifostine reduces mitochondrial membrane potential and reactive oxygen species formation in murine hepatocytes but not in hepatoma cells [64]. However, because amifostine has a short half-life and significant side effects (nausea, vomiting, and hypotension), it is rarely used clinically [61]. Theophylline is a competitive inhibitor of the adenosine receptor [65]. Adenosine reduces GFR by constricting afferent arterioles, and preclinical studies demonstrated that adenosine receptor antagonists reduced acute renal injury [66,67]. A randomized, single-blinded, placebo-controlled trial in 41 patients receiving cisplatin (50 mg/m
2) as part of their chemotherapy regimen demonstrated that theophylline preserved GFR compared to placebo-controlled subjects [65].
Whether as a single chemotherapeutic agent, in combination with other chemotherapies, or in combination with ionizing radiation, cisplatin is still considered one of the most essential and reliable treatment agents for numerous malignancies. However, cisplatin-associated toxicities, especially nephrotoxicity, can dramatically hinder individual patient clinical outcomes; therefore, research dedicated to understanding and overcoming cisplatin toxicity is critical.
- Characterization of Kidney Injury
The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines define AKI as an abrupt decrease in kidney function that occurs over a period of 7 days or less, and CKD as abnormalities in kidney structure or function that persist for >90 days [19,68]. Acute kidney disease (AKD) is described by KDIGO as acute or subacute damage or loss of kidney function for a duration of between 7 and 90 days after exposure to an AKI-initiating event [19,68]. Several definitions of AKI have been validated, including the risk, injury, failure, loss of kidney function, and end-stage kidney disease (RIFLE) classification based on serum creatinine (sCr) or urinary outputs (UO) (Table 1) [69], with the acute kidney in-jury network (AKIN) classification being based on a ≥ 50% increase in absolute sCr (1.5 × baseline value) or a decrease in UO to < 0.5 mL/kg/h for more than six hours [69]. The AKIN classification uses the staging system described in Table 1. After diagnosis of AKI by either classification, the KDIGO guidelines suggest monitoring sCr and UO for three months for resolution, new-onset, or worsening kidney dysfunction leading to chronic kidney disease (CKD) [54]. Criteria to meet the definition of CKD is determined by duration; glomerular filtration rate (GFR); and abnormal urinalysis, pathology, or structure of the kidneys [70]. CKD staging is based on GFR (mL/min/1.73 m
2. Characterization of Kidney Injury
2
2) and presence of albuminuria (Table 2). Long-term kidney dysfunction is notable in 60–80% of patients who receive cisplatin chemotherapy [71].
Table 1.AKIN vs. RIFLE classification for kidney injury based on serum creatinine (sCr) and/or urinary outputs (UO).
| UO (Common to Both) | RIFLE | ||
|---|---|---|---|
| Stage 1 Increase of ≥ 0.3 mg/dl or increase in more than or equal to 150–200% from baseline. | Less than 0.5 mg/kg/L per hour for more than 6 h | Risk Increase in sCr × 1.5 or GFR decrease >25% | |
| Stage 2 Increase to more than 200–300% from baseline. | Less than 0.5 mg/kg/L per hour for more than 12 h | Injury sCr × 2 or GFR decrease >50% | |
| Stage 3 Increased to more than 300% from baseline with an acute increase of at least 0.5 mg/dL or on RRT. | Less than 0.3 mg/kg/L for 24 h or anuria for 12 h | End-Stage Kidney Disease ESKD >3 months |
Table 2. Staging system for chronic kidney disease as per Kidney Disease Improving Global Outcomes (KDIGO) guidelines.
| GFR Stages | Kidney Function | GFR (mL/min/1.73 m2) | |||
|---|---|---|---|---|---|
| Stage G1 | Normal | ≥90 | |||
| Stage G2 | Mildly Decreased | 60–90 | Failure sCr × 3 or >4 mg/dL with an acute rise >0.5 mg/dL or GFR decrease >75% | ||
| Stage G3a | Mildly to Moderately Decreased | 45–59 | Loss Persistent acute kidney failure = complete loss of kidney function >4 weeks | ||
| Stage G3b | Moderately to Severely Decreased | 30–44 | |||
| Stage G4 | Severely Decreased | 15–29 | |||
| Stage G5 | Kidney Failure | <15 |
|
AKIN |
UO (common to both) |
RIFLE |
|
Stage 1 Increase of ≥ 0.3 mg/dl or increase in more than or equal to 150%-200% from baseline. |
Less than 0.5 mg/kg/L per hour for more than 6 hours |
Risk Increase in sCr x 1.5 or GFR decrease >25% |
|
Stage 2 Increase to more than 200%-300% from baseline. |
Less than 0.5 mg/kg/L per hour for more than 12 hours |
Injury sCr x 2 or GFR decrease >50% |
|
Stage 3 Increased to more than 300% from baseline with an acute increase of at least 0.5 mg/dL or on RRT. |
Less than 0.3 mg/kg/L for 24 hours or anuria for 12 hours |
Failure sCr x 3 or > 4 mg/dL with an acute rise >0.5 mg/dL or GFR decrease >75% |
|
|
|
Loss Persistent acute kidney failure = complete loss of kidney function > 4 weeks |
|
|
|
End-Stage Kidney Disease ESKD > 3 months |
Abbrev. AKIN, Acute Kidney Injury Network; ESKD, end-stage kidney disease; GFR, glomerular filtration rate; sCr, serum creatinine; RIFLE, risk, injury, failure, loss, and end stage; RRT, renal replacement therapy.
| AKIN |
|---|
|
GFR Stages |
Kidney Function |
GFR (mL/min/1.73m2) |
|
Stage G1 |
Normal |
≥ 90 |
|
Stage G2 |
Mildly Decreased |
60-90 |
|
Stage G3a |
Mildly to Moderately Decreased |
45-59 |
|
Stage G3b |
Moderately to Severely Decreased |
30-44 |
|
Stage G4 |
Severely Decreased |
15-29 |
|
Stage G5 |
Kidney Failure |
< 15 |
3. Pathophysiology of AKI and CKD
- Pathophysiology of AKI and CKD
While the term “AKI” is clinical, the use of acute tubular injury (ATI) is used to classify kidney injury histopathologically. In practice, ATI is semi-quantified as either mild, moderate, or severe injury and as well as focal vs. diffuse injury [72]. Characterization of kidney biopsy samples for ATI is made by the presence of tubular luminal dilation, loss of the brush border in tubules, loss of nuclei, and the presence of cytoplasmic basophilia [72]. Additionally, distinct pathological markers can be found in AKI associated with pigment administration, crystallopathy, nephrotoxic drug administration, and infection [72]. An increase in pathophysiology studies has revealed that oxidative stress, endothelial injury, mitochondrial injury, and immunological responses are key mechanisms to the AKI development of AKI. Furthermore, AKI is now considered a prominent risk factor for the CKD development of CKD, particularly in older patients and patients who have had multiple AKI episodes [72].
The definition of CKD includes not only decreases in GFR, but also structural and functional abnormalities of the kidney. Functional abnormalities such as albuminuria, proteinuria, and hematuria are classic examples. Glomerular filtration is highly dependent on high intra- and trans-glomerular pressure, which is reflected in hemodynamic injury to the kidney [73]. Additionally, CKD is promoted when the glomerular membrane’s electrostatic barrier is disrupted, allowing proteins to move into Bowman’s capsule [73]. Tubulointerstitial impairment also closely associates with long-term kidney dysfunction and encompasses many pathological features such as interstitial inflammation, kidney fibrogenesis, fibroblast activation, and promotion of the epithelial–mesenchymal transition (EMT) [73].
- Mechanism of Cisplatin-Induced Kidney Injury
5.1. Accumulation
Cisplatin uptake in the kidney is relatively unstudied and may vary between cell types. The organic cation transports (OCTs) have been implicated in the transport of cisplatin from the basolateral to the apical side in tubular cells [73,74] (Figure 1). While three isoforms of the OCT are found in the kidney, OCT2 has been found to be the largest transporter of cisplatin [73]. Upregulation of OCT2 has been shown to correlate with magnesium deficiency, which subsequently promotes the intratubular intake of cisplatin. This magnesium deficiency concurrently downregulates the multi antimicrobial extrusion protein 1 (MATE1), which is expressed at the brush-border membrane in proximal tubular cells, limiting cisplatin outtake. The combined effect of OCT2 upregulation and decreased MATE1 expression enhances cisplatin-induced AKI [75]. After cisplatin enters the tubule cells, it may undergo a variety of metabolic activations. Common pathological findings in cisplatin-treated kidney tissues are tubular cell death, apoptosis, and necrosis. Apoptosis and necrosis share similar signaling pathways, including those involved in the mitochondrial damage pathway [73]. Previous studies have shown that kidney mitochondria are the primary targets for cisplatin toxicity and that mitochondrial DNA damage drives cisplatin nephrotoxicity [25,76,77]. Mitochondria stressed by cisplatin activate caspase-mediated apoptosis by the release of caspase-9 activators. Mitochondrial DNA is also a prime target for platinum crosslinking due to the lack of efficient mitochondrial DNA repair mechanisms. This DNA is critical for encoding several inner membrane proteins including cytochrome-c oxidase subunits and ATPase [76]. Cytochrome-c oxidase (COX, complex IV) generates the proton motive force, which drives ATP production. Recent studies have shown COX enzymatic activity is weakened in proximal tubule epithelium after cisplatin treatment [78]. Furthermore, it has also been reported that this decrease in COX activity is partially due to a decrease in mitochondrial mass [79]. An early feature associated with cisplatin nephrotoxicity is oxidative stress presenting as increased 4-hydroxy-2-nonenal and increased nitro tyrosine content in mitochondrial extracts [79]. Additionally, abnormal lipid peroxidation and disruption to the synthesis of adenosine triphosphate (ATP) result in the aberrant production of free radicals and ROS.
5.2. Metabolism
Once in the kidney, cisplatin is metabolized to its active form, which is a renal toxin via a platinum-glutathione conjugate to a reactive sulfur-containing compound. This platinum-cysteine S-conjugate is bio-transformed into a reactive thiol by a pyridoxal 5’-phosphate-dependent cysteine S-conjugate β-lyase [80]. The platinum–glutathione conjugate is cleaved to a platinum–cysteinyl–glycine conjugate by gamma-glutamyl transpeptidase (GGT) on the cell surface and is subsequently cleaved to a platinum–cysteine conjugate by a dipeptidase [81,82]. The platinum–cysteine conjugate is then taken up into the cell, where it is converted to a highly reactive thiol by cysteine S-conjugate-lyase. The reactive thiol binds to cellular proteins that induced apoptosis, thereby contributing to AKI [80,83].
While the transition of AKI to CKD has yet to be illustrated, tubular cell death, oxidative distress, and vascular injury are some other mechanisms that contribute to the AKI to CKD transition in cisplatin-treated patients [73]. Further investigation into cisplatin’s nephrotoxic pathways is needed.
4. Mechanism of Cisplatin-Induced Kidney Injury
4.1. Accumulation
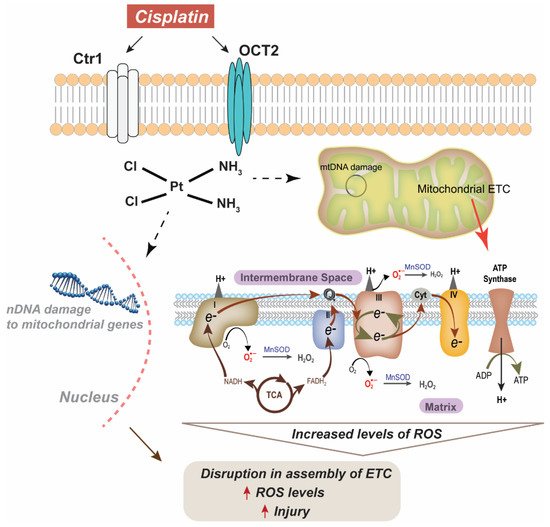
4.2. Metabolism
References
- Rosenberg, B.; VanCamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386.
- Higby, D.J.; Wallace, H.J., Jr.; Albert, D.; Holland, J.F. Diamminodichloroplatinum in the chemotherapy of testicular tumors. J. Urol. 1974, 112, 100–104.
- Einhorn, L.H.; Donohue, J. Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. Ann. Intern. Med. 1977, 87, 293–298.
- Wiltshaw, E.; Subramarian, S.; Alexopoulos, C.; Barker, G.H. Cancer of the ovary: A summary of experience with cis-dichlorodiammineplatinum(II) at the Royal Marsden Hospital. Cancer Treat. Rep. 1979, 63, 1545–1548.
- Chan, D.C. Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 2006, 22, 79–99.
- Prestayko, A.W.; D’Aoust, J.C.; Issell, B.F.; Crooke, S.T. Cisplatin (cis-diamminedichloroplatinum II). Cancer Treat. Rev. 1979, 6, 17–39.
- Rosenberg, B.; VanCamp, L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970, 30, 1799–1802.
- Harder, H.C.; Rosenberg, B. Inhibitory effects of anti-tumor platinum compounds on DNA, RNA and protein syntheses in mammalian cells in virtro. Int. J. Cancer 1970, 6, 207–216.
- Howle, J.A.; Gale, G.R. Cis-dichlorodiammineplatinum (II). Persistent and selective inhibition of deoxyribonucleic acid synthesis in vivo. Biochem. Pharmacol. 1970, 19, 2757–2762.
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320.
- Alvarez, M.V.; Cobreros, G.; Heras, A.; Lopez Zumel, M.C. Studies on cis-dichlorodiammineplatinum (II) as a radiosensitizer. Br. J. Cancer Suppl. 1978, 3, 68–72.
- Al-Sarraf, M.; Pajak, T.F.; Marcial, V.A.; Mowry, P.; Cooper, J.S.; Stetz, J.; Ensley, J.F.; Velez-Garcia, E. Concurrent radiotherapy and chemotherapy with cisplatin in inoperable squamous cell carcinoma of the head and neck. An RTOG Study. Cancer 1987, 59, 259–265.
- Schaake-Koning, C.; van den Bogaert, W.; Dalesio, O.; Festen, J.; Hoogenhout, J.; van Houtte, P.; Kirkpatrick, A.; Koolen, M.; Maat, B.; Nijs, A.; et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N. Engl. J. Med. 1992, 326, 524–530.
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153.
- Forastiere, A.A.; Goepfert, H.; Maor, M.; Pajak, T.F.; Weber, R.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; Chao, C.; et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N. Engl. J. Med. 2003, 349, 2091–2098.
- Maddalo, M.; Borghetti, P.; Tomasini, D.; Corvo, R.; Bonomo, P.; Petrucci, A.; Paiar, F.; Lastrucci, L.; Bonu, M.L.; Greco, D.; et al. Cetuximab and Radiation Therapy Versus Cisplatin and Radiation Therapy for Locally Advanced Head and Neck Cancer: Long-Term Survival and Toxicity Outcomes of a Randomized Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 469–477.
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner, H., Jr.; Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 2003, 21, 92–98.
- Faig, J.; Haughton, M.; Taylor, R.C.; D’Agostino, R.B., Jr.; Whelen, M.J.; Porosnicu Rodriguez, K.A.; Bonomi, M.; Murea, M.; Porosnicu, M. Retrospective Analysis of Cisplatin Nephrotoxicity in Patients With Head and Neck Cancer Receiving Outpatient Treatment With Concurrent High-dose Cisplatin and Radiotherapy. Am. J. Clin. Oncol. 2018, 41, 432–440.
- Ameri, A.; Norouzi, S.; Sourati, A.; Azghandi, S.; Novin, K.; Taghizadeh-Hesary, F. Randomized trial on acute toxicities of weekly vs three-weekly cisplatin-based chemoradiation in head and neck cancer. Cancer Rep. 2021, e1425.
- Reece, P.A.; Stafford, I.; Russell, J.; Khan, M.; Gill, P.G. Creatinine clearance as a predictor of ultrafilterable platinum disposition in cancer patients treated with cisplatin: Relationship between peak ultrafilterable platinum plasma levels and nephrotoxicity. J. Clin. Oncol. 1987, 5, 304–309.
- Kemp, G.; Rose, P.; Lurain, J.; Berman, M.; Manetta, A.; Roullet, B.; Homesley, H.; Belpomme, D.; Glick, J. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: Results of a randomized control trial in patients with advanced ovarian cancer. J. Clin. Oncol. 1996, 14, 2101–2112.
- Latcha, S.; Jaimes, E.A.; Patil, S.; Glezerman, I.G.; Mehta, S.; Flombaum, C.D. Long-Term Renal Outcomes after Cisplatin Treatment. Clin. J. Am. Soc. Nephrol. 2016, 11, 1173–1179.
- Stark, J.J.; Howel, S.B. Nephrotoxicity of cis-platinum (II) dichlorodiammine. Clin. Pharmacol. Ther. 1978, 23, 461–466.
- Kang, M.H.; Kang, J.H.; Song, H.N.; Jeong, B.K.; Chai, G.Y.; Kang, K.; Woo, S.H.; Park, J.J.; Kim, J.P. Concurrent Chemoradiation with Low-Dose Weekly Cisplatin in Locally Advanced Stage IV Head and Neck Squamous Cell Carcinoma. Cancer Res. Treat. 2015, 47, 441–447.
- Rossi, A.; Di Maio, M.; Chiodini, P.; Rudd, R.M.; Okamoto, H.; Skarlos, D.V.; Fruh, M.; Qian, W.; Tamura, T.; Samantas, E.; et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: The COCIS meta-analysis of individual patient data. J. Clin. Oncol. 2012, 30, 1692–1698.
- Liu, Y.H.; Li, K.; Tian, H.Q. Renoprotective Effects of a New Free Radical Scavenger, XH-003, against Cisplatin-Induced Nephrotoxicity. Oxid. Med. Cell Longev. 2020, 2020, 9820168.
- Capizzi, R.L. Amifostine reduces the incidence of cumulative nephrotoxicity from cisplatin: Laboratory and clinical aspects. Semin. Oncol. 1999, 26 (Suppl. 7), 72–81.
- Tannehill, S.P.; Mehta, M.P.; Larson, M.; Storer, B.; Pellet, J.; Kinsella, T.J.; Schiller, J.H. Effect of amifostine on toxicities associated with sequential chemotherapy and radiation therapy for unresectable non-small-cell lung cancer: Results of a phase II trial. J. Clin. Oncol. 1997, 15, 2850–2857.
- Koukourakis, M.I.; Giatromanolaki, A.; Zois, C.E.; Kalamida, D.; Pouliliou, S.; Karagounis, I.V.; Yeh, T.L.; Abboud, M.I.; Claridge, T.D.; Schofield, C.J.; et al. Normal tissue radioprotection by amifostine via Warburg-type effects. Sci. Rep. 2016, 6, 30986.
- Benoehr, P.; Krueth, P.; Bokemeyer, C.; Grenz, A.; Osswald, H.; Hartmann, J.T. Nephroprotection by theophylline in patients with cisplatin chemotherapy: A randomized, single-blinded, placebo-controlled trial. J. Am. Soc. Nephrol. 2005, 16, 452–458.
- Yao, K.; Kusaka, H.; Sano, J.; Sato, K.; Karasawa, A. Diuretic effects of KW-3902, a novel adenosine A1-receptor antagonist, in various models of acute renal failure in rats. Jpn. J. Pharmacol. 1994, 64, 281–288.
- Winston, J.A.; Safirstein, R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. Am. J. Physiol. 1985, 249, F490–F496.
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100.
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257.
- Motwani, S.S.; McMahon, G.M.; Humphreys, B.D.; Partridge, A.H.; Waikar, S.S.; Curhan, G.C. Development and Validation of a Risk Prediction Model for Acute Kidney Injury After the First Course of Cisplatin. J. Clin. Oncol. 2018, 36, 682–688.
- Section 2: AKI Definition. Kidney Int. Suppl. (2011) 2012, 2, 19–36.
- Hansen, S.W.; Groth, S.; Daugaard, G.; Rossing, N.; Rorth, M. Long-term effects on renal function and blood pressure of treatment with cisplatin, vinblastine, and bleomycin in patients with germ cell cancer. J. Clin. Oncol. 1988, 6, 1728–1731.
- Gaut, J.P.; Liapis, H. Acute kidney injury pathology and pathophysiology: A retrospective review. Clin. Kidney J. 2021, 14, 526–536.
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007.
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Yang, S.H.; Shim, H.; Cho, E.Y.; Kwon, K.B.; Kwak, T.H.; So, H.S. New Therapeutic Concept of NAD Redox Balance for Cisplatin Nephrotoxicity. Biomed. Res. Int. 2016, 2016, 4048390.
- Hamroun, A.; Lenain, R.; Bigna, J.J.; Speyer, E.; Bui, L.; Chamley, P.; Pottier, N.; Cauffiez, C.; Dewaeles, E.; Dhalluin, X.; et al. Prevention of Cisplatin-Induced Acute Kidney Injury: A Systematic Review and Meta-Analysis. Drugs 2019, 79, 1567–1582.
- Mapuskar, K.A.; Wen, H.; Holanda, D.G.; Rastogi, P.; Steinbach, E.; Han, R.; Coleman, M.C.; Attanasio, M.; Riley, D.P.; Spitz, D.R.; et al. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol. 2019, 20, 98–106.
- Singh, G. A possible cellular mechanism of cisplatin-induced nephrotoxicity. Toxicology 1989, 58, 71–80.
- Zhang, L.; Cooper, A.J.; Krasnikov, B.F.; Xu, H.; Bubber, P.; Pinto, J.T.; Gibson, G.E.; Hanigan, M.H. Cisplatin-induced toxicity is associated with platinum deposition in mouse kidney mitochondria in vivo and with selective inactivation of the alpha-ketoglutarate dehydrogenase complex in LLC-PK1 cells. Biochemistry 2006, 45, 8959–8971.
- Mukhopadhyay, P.; Horvath, B.; Zsengeller, Z.; Zielonka, J.; Tanchian, G.; Holovac, E.; Kechrid, M.; Patel, V.; Stillman, I.E.; Parikh, S.M.; et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic. Biol. Med. 2012, 52, 497–506.
- Zsengeller, Z.K.; Ellezian, L.; Brown, D.; Horvath, B.; Mukhopadhyay, P.; Kalyanaraman, B.; Parikh, S.M.; Karumanchi, S.A.; Stillman, I.E.; Pacher, P. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J. Histochem. Cytochem. 2012, 60, 521–529.
- Cooper, A.J.; Krasnikov, B.F.; Niatsetskaya, Z.V.; Pinto, J.T.; Callery, P.S.; Villar, M.T.; Artigues, A.; Bruschi, S.A. Cysteine S-conjugate beta-lyases: Important roles in the metabolism of naturally occurring sulfur and selenium-containing compounds, xenobiotics and anticancer agents. Amino Acids 2011, 41, 7–27.
- Hanigan, M.H.; Lykissa, E.D.; Townsend, D.M.; Ou, C.N.; Barrios, R.; Lieberman, M.W. Gamma-glutamyl transpeptidase-deficient mice are resistant to the nephrotoxic effects of cisplatin. Am. J. Pathol. 2001, 159, 1889–1894.
- Townsend, D.M.; Deng, M.; Zhang, L.; Lapus, M.G.; Hanigan, M.H. Metabolism of Cisplatin to a nephrotoxin in proximal tubule cells. J. Am. Soc. Nephrol. 2003, 14, 1–10.
- Zhang, L.; Hanigan, M.H. Role of cysteine S-conjugate beta-lyase in the metabolism of cisplatin. J. Pharmacol. Exp. Ther. 2003, 306, 988–994.
