Deoxyribonucleic acid (DNA), a genetic material, encodes all living information and living characteristics, e.g., in cell, DNA signaling circuits control the transcription activities of specific genes. In recent years, various DNA circuits have been developed to implement a wide range of signaling and for regulating gene network functions. In particular, a synthetic DNA circuit, with a programmable design and easy construction, has become a crucial method through which to simulate and regulate DNA signaling networks. Importantly, the construction of a hierarchical DNA circuit provides a useful tool for regulating gene networks and for processing molecular information. Moreover, via their robust and modular properties, DNA circuits can amplify weak signals and establish programmable cascade systems, which are particularly suitable for the applications of biosensing and detecting. Furthermore, a biological enzyme can also be used to provide diverse circuit regulation elements.
- synthetic DNA circuit
- DNA strand displacement
- DNA self-assembly
- DNA networks
1. Introduction
Recently, various artificial DNA circuits have been established and widely applied to many fields such as medical diagnosis [1][2][3][1,2,3], molecular detection, and information processing [4][5][6][7][8][9][10][4,5,6,7,8,9,10]. Particularly, synthetic DNA circuits, designed and constructed in vitro, perform an important role in effectively controlling the gene networks in cell [11][12][13][11,12,13]. Synthetic DNA circuits have been demonstrated as possessing superiority in simulating and regulating DNA signaling, due to the properties of programmability and easy operation [14][15][16][17][18][14,15,16,17,18]. More importantly, synthetic DNA circuits have the potential to promote complex biological information processes and provide a new way to achieve gene analysis and molecular information processing [19][20][21][19,20,21].
Using predesigned specific base pair recognition, synthetic DNA circuits can modulate complex gene networks to implement diverse biofunctions. Recently, a variety of bioengineering and biocomputing functions have been regulated by varying the architectures and integrations of DNA circuits, such as their signal simulation [5][22][5,22], the molecular switch, catalytic cycle, cascade amplification [23][24][25][26][27][28][29][30][23,24,25,26,27,28,29,30], and logic gates [31][32][33][34][35][36][37][31,32,33,34,35,36,37]. In fact, most of the DNA circuits are implemented and regulated for the DNA strand displacement, whereby the longer DNA strand is able to hybridize with the complementary strand to displace the shorter one [38][39][40][38,39,40]. Through a DNA strand displacement reaction (SDR), a synthetic DNA circuit can be used to precisely regulate complex gene networks and molecular biosystems, e.g., DNA neural network systems that are constructed to implement pattern recognition [41].
2. The Research Studies on DNA Circuits
2.1. Cascading DNA Circuits In Vitro
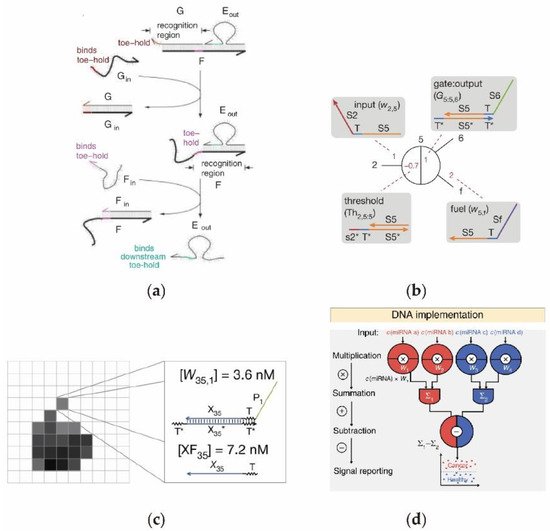
2.2. Enzyme-Free Catalytic DNA Circuit
2.2. Enzyme-Free Catalytic DNA Circuit
In natural gene networks, protein enzymes are typically used as catalysts, while DNA seldom performs a catalyzing function. This means that the input DNA signals are continuously consumed in a normal noncatalytic DNA circuit reaction, thus leading to a limited reaction efficiency. In synthetic DNA circuit, by introducing a recyclable DNA as a catalyst the reaction efficiency is greatly improved, and only a small amount of input DNA is required to release a large amount of output DNA [48][56]. Therefore, a catalytic DNA circuit can be used to construct a more efficient artificial nucleic acid system without the use of a bioenzyme. In 2006, Seelig et al. [49][57] first proposed a DNA circuit with high catalytic efficiency based on DNA strand replacement by establishing a catalytic cycling unit. Figure 2a illustrates the operation of this enzyme-free catalytic circuit based on the principle of DNA displacement. Since the linear DNA structure is more stable and easily transformed, Zhang et al. [50][58] designed an enzyme-free DNA circuit with a high catalytic performance, using single-stranded DNA as a catalyst. As shown in Figure 2b, the DNA catalyst was used repeatedly in the catalytic cycle without being consumed, and a large amount of outputDNA was produced along with the generation of waste and intermediate products. Zhang et al. [23] introduced an inhibitor DNA and activator DNA into the catalytic system. As shown in Figure 2c, in contrast to a simple linear ssDNA catalyst, a loop DNA catalyst can be used to perform the catalytic function, where the catalytic process can be dynamically switched according to the specific inhibitors and activators. By combining the enzyme-free catalytic circuit with DNA tile technology, David Yu Zhang et.al realized the rapid and large-scale assembly of micro-nanotubes [51][59]. The linear catalytic circuit in Figure 2d continuously generates the single-stranded DNA required to assemble large-scale nanotubes, thereby achieving the function of efficiently increasing the size of nanotubes within a short period.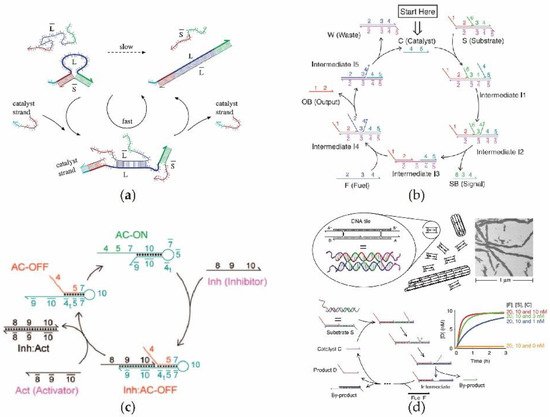
.3. DNAzyme-Based DNA Circuit
2.3. DNAzyme-Based DNA Circuit
DNAzyme is a specific single-stranded DNA fragment with a catalytic cutting function, high catalytic activity, and specific recognition capacity. One of its most important roles is to cleave the RNA site via esterification. By taking advantage of this property, the DNA circuit can directly employ DNAzyme to accurately control its circuit structure and signal transmission mode [52][53][54][60,61,62]. On the other hand, the structure and activity of DNAzyme can be precisely regulated, thus providing the stronger controllability for DNA circuits [27][55][56][57][58][59][60][27,63,64,65,66,67,68]. Utilizing a metal-ion ion dependent DNAzyme, Moshe et al. [61][69] constructed a DNA circuit that was regulated by uranium dioxide and magnesium. As shown in Figure 3a, only once both DNAzymes were activated simultaneously could the target DNA sequence be released. Moreover, by combining gold nanoparticles with DNAzyme, Bi et al. [62][70] designed a DNAzyme logic circuit characterized by colorimetic and UV/vis detection. As illustrated in Figure 3b, circle DNA with different RNA modification sites can be cut into several DNA fragments by the DNAzyme, resulting in the aggregation of gold nanoparticles. The potential of the upstream output signal being successfully transmitted to the downstream as an input signal without a signal leakage in the process of transmission remains a major challenge in DNAzyme cascade circuits. Unlike the traditional DNAzyme with fixed structures, Elbaz et al. [63][71] constructed a DNA circuit with a controllable DNAzyme by splicing the structure into a hybrid structure (Figure 3c). The signal catalytic function of DNAzyme provides the advantage reusability in the process of RNA cleavage, however, it also causes the DNAzyme-based circuit to become uncontrollable, which greatly limits the time domain characteristics of the circuits. Compared with the previous unchangeable DNAzyme, Harding et al. [64][73] designed a DNA circuit using the DNAzyme with a dynamic switching ability (Figure 3d). Therefore, the activity of the DNAzyme can be well regulated using an additional input DNA signal.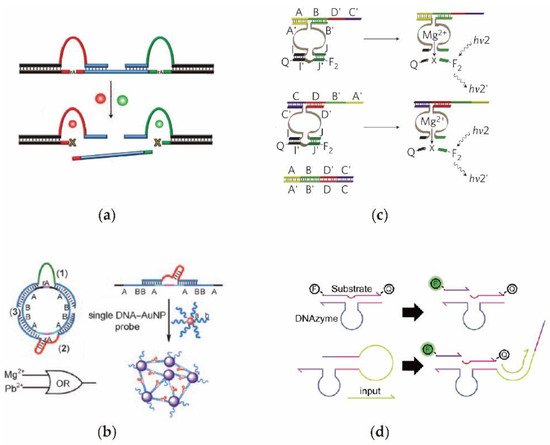
2.4. Protein Enzyme-Assiste d DNA Circuit
A biological enzyme is a type of biomolecule that can specifically recognize the substrate to perform its catalytic function [65][74]. According to their catalytic reaction properties, biological enzymes can be generally divided into categories such as oxidoreductase, transferases, and hydrolases. Due to its high specificity, strong catalytic ability, and fast reaction speed, the enzyme-assisted DNA circuit is suitable for building a more intelligent biological computing system. Recently, biological enzymes have been used to build functional DNA circuits [66][67][68][75,76,77]. Through their simulation of ecosystems, Fuji et al. [5] designed an enzyme-assisted DNA circuit with oscillating and competitive functions (Figure 4a). In the system, both the predator P and prey N were dynamically generated and degraded in the presence of the polymerase, nicking enzyme, and exonuclease. In 2017, inspired by signaling networks in living cells, Lenny et al. [69][78] designed an enzyme-driven DNA circuit that can dynamically regulate the signal strength during a reaction (Figure 4b). The researchers applied this toolbox to various DNA circuits to realize the bionic electronic functions by using DNA as the information carrier. Song et al. [70][42] established a logic DNA circuit composed of simple ssDNA and polymerase-triggered DNA strand displacement (Figure 4c). Significantly, since the DNA circuit consists of only single-stranded DNA, the problems of signal leakage and signal reset are resolved well. Through the combination of the enzyme- and entropy-driven DNA catalytic reaction, Zhang et al. [71][79] constructed a dual-catalytic recyclable DNA circuit (Figure 4d).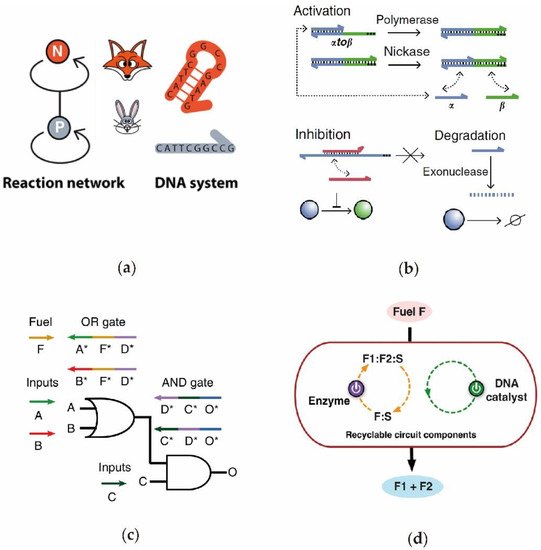
2.5. The DNA Circuits on Origami Surface
2.5. The DNA Circuits on Origami Surface
A DNA origami assembly was developed by Rohemund in 2006, in which hundreds of short DNA fold with a M13mp18 scaffold to construct various nanometer shapes, such as squares, circles, and triangles. As the surface of DNA origami can modify DNA in a practicable way, DNA circuits can be constructed onto the origami surface to achieve vector-based logic operations and robot movements [72][73][74][75][76][77][43,80,81,82,83,84]. Such an on-surface DNA circuit operation is a new method of gene regulation, exhibiting the properties of specific spatial designs and modular constructions. As shown in Figure 5a, by utilizing synthetic molecular motors that move autonomously along liner tracks, Shelley et al. [78][85] designed a programmed network system on the surface of a rectangular DNA origami. After moving, the majority of motors can follow the correct path to the predesigned target aim. This programmable on-surface DNA circuit motor has the potential to build complex and controllable information transmission systems. In 2014, to investigate the kinetic and efficiency of on-surface DNA circuit, Teichmann et al. [79][86] installed DNA circuit units of signal sender and receiver on an origami surface (Figure 5b). In the on-surface circuit, the sender unit was situated in the middle (red color point) of the origami platform and the receiver units were distributed evenly on the plane around the sender. Interestingly, researchers can regulate the signal transmission by adjusting the distributions of gate units, so as to optimize the efficiency and accuracy of the on-surface DNA circuits. In addition, the precise arrangement of on-surface circuit is of great significance for DNA-based memories and information processing. In 2017, Winfree developed a DNA robot with the ability to carry cargo in sequence on the origami surface by designing a randomly moving DNA circuit [80][87] (Figure 5c). Gourab et al. [42][49] constructed a surface logic DNA circuit whereby the signal transmission path can be programmed to realize the directional logic operation as shown in Figure 5d.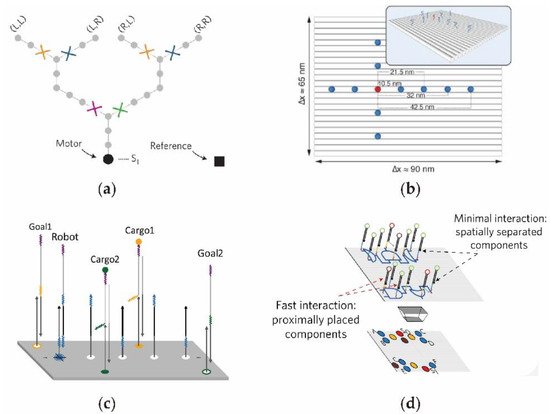
2.6. The DNA Circuits Combined with Nanoparticles
2.6. The DNA Circuits Combined with Nanoparticles
Compared to other macroscopic materials, nanoparticles possess the advantages of well-controlled sizes and shapes [81][82][83][45,88,89], visible spectroscopy characteristics [84][85][86][87][90,91,92,93], and multiple chemical modifications. Therefore, nanoparticles have been widely used in multiple disciplines, e.g., in medicine, bioengineering, and molecular signal detection [88][89][90][91][92][94,95,96,97,98]. Recently, combined with a DNA circuit, nanoparticles have been widely applied to perform the functions of biosensing, molecular signal detection, and DNA computing [93][94][95][96][99,100,101,102].3. Summary
Although DNA circuits have engendered remarkable progresses in recent years, there are still some critical aspects that need to be improved upon to broaden the related application fields. The most important aspect is the signal leakage and distortion that exists in various complex DNA circuits [41][46][41,54]. The primary reason for this phenomenon is that some redundant base complementary pairings easily emerged during the circuit operations. At present, the design methods implemented to program DNA sequences are mainly based on the combinations of a manual design and simple software. Therefore, it is still difficult to completely eliminate these interference signals using pre-established methods, and a more automatic and universal sequence design platform is eagerly desired in the future. In addition, another important consideration to account for is the method by which to ensure the DNA circuit is reusable, thereby promoting its practical applications. Compared with the repeated electronic circuits, DNA circuits cannot be reused after completing the first complete operation [70][47][97][42,55,108], which inhibits the subsequent development of more advanced intelligent DNA circuits. Specifically, in large computing DNA circuits, it is important to establish a reusable circuit mechanism, to meet requirements of frequently adjusted circuit parameters and to test the structural stability of the system. In fact, many studies have been undertaken to improve the repeatability of the DNA circuits. More recently, Zhang et al. reported a nicking-assisted recycling strategy for implement reusable DNA circuits to achieve a repeatability of over 20 times during one reaction cycle [71][79].
