(1) In a cafeteria experiment, beavers cThose P. fremontii six times more often than other woody native and exotic species. (2) With two cottonwood species, we found that the nitrogen and salicortin concentrations were up to 45% greater and lignin concentration 14% lower in the juvenile resprout growth of felled trees than the juvenile growth on unfelled trees (six of seven analyses were significant for P. fremontii and four of six were significant for P. angustifolia). (3) With two cottonwood species, arthropod community composition on juvenile branches differed significantly between felled and unfelled trees, with up to 38% greater species richness, 114% greater relative abundance and 1282% greater species diversity on felled trees (six of seven analyses with P. fremontii and four of six analyses with P. angustifolia were significant). The above findings indicate that the highest arthropod diversity is achieved in the heterogenous stands of mixed felled and unfelled trees than in stands of cottonwoods, where beavers are not present. These results also indicate that beaver herbivory changes the chemical composition in 10 out of 13 chemical traits in the juvenile growth of two of the three cottonwood species to potentially allow better defense against future beaver herbivory. (4) With P. deltoides, only one of five analyses in chemistry was significant, and none of the four arthropod e North American beaver (Castor canadensis Kuhl) and cottonwoods (Populus spp.) are foundation species, the interactions of which define a much larger community analyses were significant, suggesting that this species and its arthropod community responds differently to beaver. Potential reasons for these differences are unknown. Overall, our findings suggest that in addition to their impact on riparian vegetation, other mammals, birds, and aquatic organisms, beavers also may define the arthropod communities of two of three foundation tree species in these riparian ecosystemsd affect a threatened riparian habitat type.
- beaver
- Castor canadensis
- cottonwood
- Populus spp.
- chemistry
- arthropod communities
- foundation species
- ecology
1. Introduction
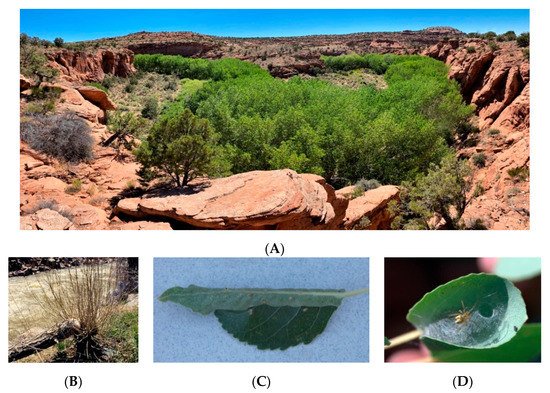
2. Beaver Cafeteria Feeding Experiment
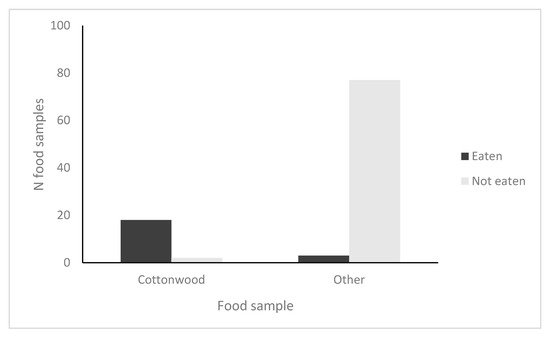
3. Tree Phytochemical Responses to Beaver Herbivory
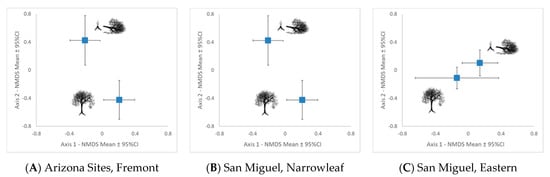
4. Arthropod Responses to Herbivory
5. Conclusions
Our study found patterns largely in agreement with our hypotheses that beavers preferentially choose cottonwoods over other tree species, impacting cottonwood twig chemistry and influencing arboreal arthropod communities: (1) When offered a variety of locally occurring woody plant species, beavers preferentially selected Fremont cottonwood six times more often than other available food samples; (2) Twig tissue from resprout growth of felled trees showed different defensive, structural and nutritional phytochemistry concentrations than twig tissue from paired unfelled trees; and (3) Juvenile resprout growth on beaver-felled trees supported significantly different arthropod communities, often with higher species richness, diversity and abundance than the juvenile growth of unfelled cottonwoods. This trio of patterns was repeated in both Fremont and narrowleaf cottonwood stands, arguing that cottonwood stands with heterogeneity resulting from beaver felling support higher arthropod diversity than cottonwood stands without beaver influence. These alterations to the phytochemistry of juvenile resprout growth following beaver felling may impact future herbivory. Strikingly, Eastern cottonwood did not follow these patterns, and we are unaware of studies that have investigated reasons for this different response. Our findings indicate that as interacting foundation species, beavers and cottonwoods can impact the phytochemistry of riparian tree stands as well as the community structure of arthropods on these trees. As riparian habitat becomes ever more imperiled due to climate change, invasive species, and development, our understanding of these interactions and their community-wide impacts are increasingly critical for conservation and management. As we further our understanding of these complex and fragile ecosystems, we can be better prepared to prioritize research and responses that protect and restore functionality for biotic communities and ecosystem processes.References
- Holling, C.S. Cross-Scale Morphology, Geometry, and Dynamics of Ecosystems. Ecol. Monogr. 1992, 62, 447–502.
- Dayton, P.K. Toward an Understanding of Community Resilience and the Potential Effects of Enrichments to the Benthos at McMurdo Sound, Antarctica. In Proceedings of the Colloquium on Conservation Problems in Antarctica; Parker, B.C., Ed.; Allen Press: Lawrence, KS, USA, 1972; pp. 81–96.
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliot, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486.
- Whitham, T.G.; Allan, G.J.; Cooper, H.F.; Shuster, S.M. Intraspecific genetic variation and species interactions contribute to community evolution. Annu. Rev. Ecol. Syst. 2020, 51, 587–612.
- Keith, A.R.; Bailey, J.K.; Whitham, T.G. A genetic basis to community repeatability and stability. Ecology 2010, 11, 3398–3406.
- Keith, A.R.; Bailey, J.K.; Lau, M.K.; Whitham, T.G. Genetics-based interactions of foundation species affect community diversity, stability, and network structure. Proc. Biol. Sci. 2017, 284, 20162703.
- Hagglund, A.; Sjoberg, G. Effects of beaver dams on the fish fauna of forest streams. For. Ecol. Manag. 1999, 115, 259–266.
- Hammerson, G.A. Beaver (Castor canadensis): Ecosystem alterations, management, and monitoring. Nat. Area J. 1994, 14, 44–57.
- Rosell, F.; Bozsér, O.; Collen, P.; Parker, H. Ecological impact of beavers Castor fiber and Castor Canadensis and their ability to modify ecosystems. Mammal Rev. 2005, 35, 248–276.
- Naiman, R.J.; Pinay, G.; Johnston, C.A.; Pastor, J. Beaver influences on the long-term biogeochemical characteristics of boreal forest drainage networks. Ecology 1994, 75, 905–921.
- Ecke, F.; Levanoni, O.; Audet, J.; Carlson, P.; Eklof, K.; Hartman, G.; McKie, B.; Ledsma, J.; Segersten, J.; Truchy, A.; et al. Meta-analysis of environmental effects of beaver in relation to artificial dams. Environ. Res. Lett. 2017, 12, 11302.
- Martinsen, G.D.; Driebe, E.M.; Whitham, T.G. Indirect interactions mediated by changing plant chemistry: Beaver browsing benefits beetles. Ecology 1998, 79, 192–200.
- Bailey, J.K.; Whitham, T.G. Interactions between cottonwood and beavers positively affect sawfly abundance. Eco. Entomol. 2006, 31, 294–297.
- Bailey, J.K.; Whitham, T.G. Biodiversity is Related to Indirect Interactions among Species of Large Effect. In Ecological Communities: Plant Mediation in Indirect Interaction Webs; Ohgushi, T., Craig, T.P., Price, P.W., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 306–328.
- Bailey, J.K.; Schweitzer, J.A.; Rehill, B.J.; Lindroth, R.L.; Martinsen, G.D.; Whitham, T.G. Beavers as molecular geneticists: A genetic basis to the foraging of an ecosystem engineer. Ecology 2004, 85, 603–608.
- Strzelec, M.; Białek, K.; Spyra, A. Activity of beavers as an ecological factor that affects the benthos of small rivers—A case study in the Żylica River (Poland). Biologia 2018, 73, 577–588.
- Mitchell, C.C. Vegetation change in a topogenic bog following beaver activity. Bull. Torrey Bot. Club 1999, 120, 136–147.
- McGinley, A.; Whitham, T.G. Central place foraging by beavers (Castor canadensis): A test of foraging predictions and the impact of selective feeding on the growth form of cottonwoods (Populus fremontii). Oecologia 1985, 66, 558–562.
- Walker, F.M.; Durben, R.; Shuster, S.M.; Lindroth, R.L.; Whitham, T.G. Heterozygous trees rebound fastest after felling by beavers to positively affect arthropod community diversity. Forests 2021, 12, 694.
- Johnston, C.A.; Naiman, R.J. The use of a geographic information system to analyze long-term landscape alteration by beaver. Landsc. Ecol. 1990, 4, 5–19.
- Chadde, S.W.; Kay, C.E. Tall Willow Communities on Yellowstone’s Northern Range: A test of the ‘‘Natural Regulation’’ Paradigm. In The Greater Yellowstone Ecosystem; Keiter, R.B., Boyce, M.S., Eds.; Yale University Press: New Haven, CT, USA, 1991; pp. 231–262.
- Wright, J.; Chambers, J. Restoring riparian meadows currently dominated by Artemisa using alternative state concepts—Above-ground vegetation response. Appl. Veg. Sci. 2002, 5, 237–246.
- Stevens, C.E.; Paszkowski, C.A.; Scrimgeour, G.J. Older is better: Beaver ponds on boreal streams as breeding habitat for the wood frog. J. Wildl. Manag. 2006, 70, 1360–1371.
- Naiman, R.J.; Decamps, H.; Pollack, M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993, 3, 209–212.
- Farley, G.; Ellis, L.; Stuart, J.; Scott, N. Avian species richness in different-aged stands of riparian forest along the Middle Rio Grande, New Mexico. Conserv. Biol. 1994, 8, 1098–1108.
- Zimmerman, J.C.; DeWald, L.E.; Rowlands, P.G. Vegetation diversity in an interconnected ephemeral riparian system of north-central Arizona, USA. Biol. Conserv. 1999, 90, 217–228.
- Jenkins, S.H.; Busher, P.E. Castor canadensis. Mamm. Species 1979, 120, 1–8.
- Rohlf, F.J.; Sokal, R.R. Statistical Tables, 3rd ed.; W. H. Freeman and Company: San Francisco, CA, USA,, 1995.
