Oral mucositis (OM) is a pathological condition with several oral manifestations, originate from cytotoxic effects of non-surgical cancer therapies. The clinical manifestation ranges from diffuse erythematous areas to necrotic ulcers lesions in the mucosa. The oral mucosa presents confluent, deep, and devastatingly painful ulcerations in the most advanced clinical form.[1] Almost all oral or oropharyngeal mucosa areas undergoing radiation will develop this side effect, however, the patients undergoing chemotherapy regiment develop the condition depending on the dose and cytotoxicity of the drug used. Usually, incidence goes around 20 to 40% for solid tumors, while in the therapies with a high dose of cytotoxic drugs, like hematopoietic stem cell transplant, the incidence is around 80%.[2] The patients that develop OM during the cancer treatment can manifest alterations in physical, mental, emotional, and social health factors, proving an unhealthy state. Patients present diet modifications and weight loss, necessitate opioid analgesics, require supplemental nutrition, increase the risk of bacteremia and sepsis, disrupt optimal cancer therapy, and increase healthcare costs. [3]
It is common the association of head and neck cancer and OM in medical care however, the frequency in other cancers has long been overlooked and underreported. For this reason, a multidisciplinary team composed of other health professionals, as dentists, can identify and treat pathologies in advance during oncological treatment.
The OM development is described in a five-step pathogenesis model with several biological factors’ combinations. The lesion occurs with the damage of basal epithelial cells due to the radio-chemotherapy. The cascade of events starts with severe alterations in the environment that involves the generation of reactive oxygen species (ROS), activation of transcription factors (NF-kB) and inflammatory pathways (COX), and up-regulation of proinflammatory cytokines (TNF-a, IL1b, and IL-6).[2]
The clinical diagnosis can be made in the early stages. The mucosa presents erythema, the patients complain of burning and intolerance of some specific foods. After two weeks, the ulcerated lesions can be detected in one or more areas of the oral cavity. The patient refers to slight discomfort and inconvenience to severe pain, dysphagia, and difficulty in eating that lead to the opioid intervention. As a result of the cancer treatment, it is common to occur salivary alterations in composition and quantity, leading to the exasperation of OM development and impairment in the patient’s quality of life. The lesions recover depending on the patient's immune compromise, however, heal spontaneously for at least 2 weeks following the completion of the therapeutic regimen.[4] Medical and scientific community discourse about effective management of OM in cancer patients due to its high incidence and clinical significance in patient prognosis. Several scientific studies are carried out to discover a well-defined strategy that provides improved management of OM that may allow more aggressive therapeutic doses and more effective cancer treatment, improved patient survival, and wellbeing.[5]
All guidelines for the management of OM agree OM management can be divided into three basic components: general oral care, prevention, and palliative cares. The oral care purpose is to reduce some host-related risk factors for stomatitis, including lowering the impact of oral microbial flora. Therefore, a simple care protocol must be suggested, as brushing teeth, daily flossing, and mouth rinsing. In addition, spicy food, alcoholic beverages, and alcohol-based mouthwashes must be avoided.[6]
Prevention is the second most important factor in addressing oral mucositis. The combination of agents and physical strategies can provide anti-inflammatory, analgesic, and anti-microbial more effective effects in OM management. The preventive use of oral cryotherapy and photobiomodulation (PBM) therapy showed a reduction in the impact of the treatment toxicity in the oral mucosa.[7] The OM treatment effectivity increase can be noted with the use of several pharmacological agents (pentoxifylline, benzydamine hydrochloride, thalidomide, and simvastatin) and natural products such as Omega-3 FFA, essential oils from manuka (Leptospermum scoparium), vitamins, glutamine, chamomile, aloe vera, and curcumin.[8] The OM palliative care has focused on symptom control using topical or systemic analgesics and the application of barrier agents to cover injured mucosa.[3]
In conclusion, OM is a painful and wasting consequence of anticancer chemotherapy and/or radiotherapy. The occurrence of this pathology increases the risk of treatment interruption and a decrease in quality of life. A multidisciplinary team can provide global attention during the treatment, detecting early necessary interventions to manage the side effects of the cytotoxic therapeutic and providing wellbeing for cancer patients.
Reference:
1. Sonis, S. Oral Mucositis. Volume1. Springer Healthcare 2012 ISBN 10.1007/978-1-907673-46-7.
2. Sollecito,PT. Clinical Approaches to Oral Mucosal Disorders: Part II, An Issue of Dental Clinics of North America, Volume 58, Issue 2 of The Clinics: Dentistry, Elsevier Health Sciences, 2014, ISBN 0323289967, 9780323289962).
3. Sonis, S.T. Treatment for Oral Mucositis—Current Options and an Update of Small Molecules Under Development. Current Treatment Options in Oncology. 2021 22.. doi:10.1007/s11864-021-00823-6).
4. (Scully, C., Sonis, S., Diz, P., 2006. Oral mucositis. Oral Diseases 12, 229–241. doi:10.1111/j.1601-0825.2006.01258.x).
5. Campos MI, Campos CN, Aarestrup FM, Aarestrup BJ. Oral mucositis in cancer treatment: Natural history, prevention and treatment. Mol Clin Oncol. 2014;2(3):337-340. doi:10.3892/mco.2014.2536.
6. Lionel D, Christophe L, Marc A, Jean-Luc C. Oral mucositis induced by anticancer treatments: physiopathology and treatments. Ther Clin Risk Manag. 2006;2(2):159-168. doi:10.2147/tcrm.2006.2.2.159
7. de Carvalho, P.A.G.; Lessa, R.C.; Carraro, D.M.; Assis Pellizzon, A.C.; Jaguar, G.C.; Alves, F.A. Three photobiomodulation protocols in the prevention/treatment of radiotherapy-induced oral mucositis. Photodiagnosis Photodyn Ther. 2020 Sep; 31:101906. doi: 10.1016/j.pdpdt.2020.101906.
8. Lessa, R.C.; Alves, F.A.; Fortunati E.; Lu J. Oral Mucositis in Cancer and Potential Use of Omega-3 Free Fatty Acids in Its Management: A Review Biomedicines 2021 doi: 10.3390/biomedicines9111531.
- oral mucositis
- inflammation
- polyunsaturated fatty acids
- omega-3 fatty acids
1. Introduction
2. Biomolecular Mechanisms of OM
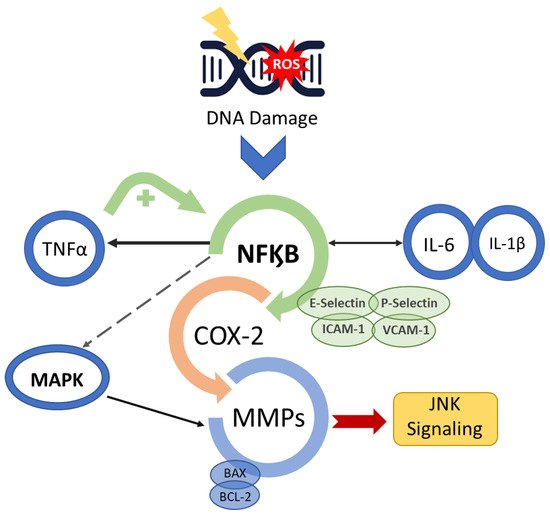
Radiation-induced and chemotherapy-induced OM have similar developmental mechanisms [1]. The cascade of biological events responsible for the genesis of OM begins with the induction of DNA damage caused by radiation or chemotherapeutic cancer therapy [6,13][5][6] (Figure 1).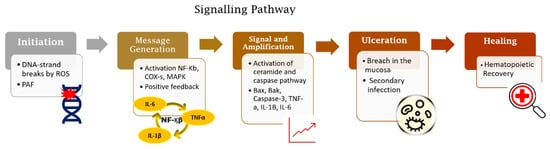
3. Prevention and Management Strategies
OM treatment is a miscellany of therapies that quest the control of the diseases and the symptoms relieved. Therefore, the clinical practice guidelines from the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) summarized the standard protocol to manage the OM in cancer patients [32,34][26][28]. The proposed intervention strategy is to begin with the care in oral health with a combination of toothbrushing and flossing [35][29]. The strategy is followed by the elimination of any type of irritant and the control of the proliferation of oral pathogenic microflora through mechanical removal, combined with the individual use of mouth rinses to maintain oral hygiene. Complete oral examinations and dental interventions are critical components performed in conjunction with oncological treatments [32,34,35][26][28][29]. Oral cryotherapy and photobiomodulation (PBM) therapy have been utilised preventively to reduce the impact of the treatment toxicity in the oral mucosa [36][30]. The PBM is recommended for the prevention and treatment of OM in patients receiving cancer treatments. Several studies have demonstrated the effectiveness of anti-inflammatory effects in supporting tissue repair [13,33,37,38,39,40,41,42,43,44,45][6][27][31][32][33][34][35][36][37][38][39]. Nevertheless, clinical evidence still shows that some patients present recurring episodes of OM during their cancer therapy despite being treated with LLLT [37][31]. Pharmacological agents (pentoxifylline, benzydamine hydrochloride, thalidomide and simvastatin) currently utilized to prevent and treat OM have variable efficacy rates and significant side effects, rendering this treatment strategy less than ideal [13,14,15][6][7][8]. The requirement to reduce the side effects of pharmacological agents and increase the possibility of a patient’s fast recovery elicited the need for research to demonstrate the benefits of utilising natural resources and herbal medicines to manage the OM wound and related inflammatory conditions [3,33][3][27]. For this reason, several natural products such as chamomile, essentials oils from manuka (Leptospermum scoparium) and kanuka (Kunzea ericoides), vitamins A, B12 and E, folate, glutamine, aloe vera and curcumin have been studied [46,47,48,49][40][41][42][43]. In addition, several studies have investigated the mechanisms of action of n-3 fatty acids (or omega-3 fatty acids) against several diseases, with observed successes that are likely due to the fatty acids’ anti-inflammatory effects [50][44]. Researchers have demonstrated that the use of a combination of agents and physical strategies can provide anti-inflammatory, analgesic and anti-microbial effects that can be used to manage cancer-therapy-induced OM in general. The combination strategy has been promising for patients’ symptom relief and wellness during the OM course [13,37][6][31].References
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77.
- Nonzee, N.J.; Dandade, N.A.; Patel, U.; Markossian, T.; Agulnik, M.; Argiris, A.; Patel, J.D.; Kern, R.C.; Munshi, H.G.; Calhoun, E.A.; et al. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis: Results from a Northwestern University Costs of Cancer Program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer 2008, 113, 1446–14452.
- Alvariño-Martín, C.; Sarrión-Pérez, M.G. Prevention and treatment of oral mucositis in patients receiving chemotherapy. J. Clin. Exp. Dent. 2014, 6, e74–e80.
- De Barros, P.A.V.; Rabelo Andrade, M.E.; de Vasconcelos Generoso, S.; Mendes Miranda, S.E.; Dos Reis, D.C.; Lacerda Leocádio, P.C.; de Sales E Souza, É.L.; Dos Santos Martins, F.; da Gama, M.A.S.; Cassali, G.D.; et al. Conjugated linoleic acid prevents damage caused by intestinal mucositis induced by 5-fluorouracil in an experimental model. Biomed. Pharmacother. 2018, 103, 1567–1576.
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284.
- Treister, N.; Sonis, S. Mucositis: Biology and management. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 123–129.
- Sonis, S.T. A biological approach to mucositis. J. Supp. Oncol. 2004, 2, 21–32.
- Shankar, A.; Roy, S.; Bhandari, M.; Rath, G.K.; Biswas, A.S.; Kanodia, R.; Adhikari, N.; Sachan, R. Current Trends in Management of Oral Mucositis in Cancer Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 2019–2026.
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164.
- Blijlevens, N.; Sonis, S. Palifermin (recombinant keratinocyte growth factor-1): A pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann. Oncol. 2007, 18, 817–826.
- Razmara, F.; Khayamzadeh, M. An Investigation into the Prevalence and Treatment of Oral Mucositis After Cancer Treatment. Int. J. Cancer Manag. 2019, 12, e88405.
- Logan, R.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. Nuclear factor-κB (NF-κB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007, 43, 395–401.
- Lionel, D.; Christophe, L.; Marc, A.; Jean-Luc, C. Oral mucositis induced by anticancer treatments: Physiopathology and treatments. Ther. Clin. Risk. Manag. 2006, 2, 159–168.
- Mafra, C.A.D.C.C.; Vasconcelos, R.C.; de Medeiros, C.A.C.X.; Leitão, R.F.C.; Brito, G.A.C.; Costa, D.V.D.S.; Guerra, G.C.B.; de Araújo, R.F., Jr.; Medeiros, A.C.; de Araújo, A.A. Gliclazide Prevents 5-FU-Induced Oral Mucositis by Reducing Oxidative Stress, Inflammation, and P-Selectin Adhesion Molecules. Front. Physiol. 2019, 10, 327.
- Sonis, S.T. The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit. Rev. Oral Biol. Med. 2002, 13, 380–389.
- Al-Dasooqi, N.; Gibson, R.J.; Bowen, J.M.; Logan, R.M.; Stringer, A.M.; Keefe, D.M. Matrix metalloproteinases are possible mediators for the development of alimentary tract mucositis in the dark agouti rat. Exp. Biol. Med. 2010, 235, 1244–1256.
- Al-Dasooqi, N.; Sonis, S.T.; Bowen, J.M.; Bateman, E.; Blijlevens, N.; Gibson, R.J.; Logan, R.M.; Nair, R.G.; Stringer, A.M.; Yazbeck, R.; et al. Mucositis Study Group of Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Emerging evidence on the pathobiology of mucositis. Supportive Care Cancer 2013, 21, 2075–2083.
- Sonis, S.T. Pathobiology of oral mucositis: Novel insights and opportunities. J. Support Oncol. 2007, 5, 3–11.
- Vesty, A.; Gear, K.; Biswas, K.; Mackenzie, B.W.; Taylor, M.W.; Douglas, R.G. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Supportive Care Cancer 2020, 28, 2683–2691.
- Haverman, T.M.; Laheij, A.M.G.A.; Nie, M.; Deng, D.M.; Raber-Durlacher, J.E.; de Soet, J.J.; Rozema, F.R. Exploring the role of oral microorganisms in the pathogenesis of mucositis by assessing their impact on metabolic activity and reproductive capacity of epithelial cells in vitro. Supportive Care Cancer 2020, 28, 4729–4735.
- Laheij, A.M.; de Soet, J.J. Can the oral microflora affect oral ulcerative mucositis? Curr. Opin. Supportive Palliat. Care 2014, 8, 180–187.
- Van Saene, H.K.; Martin, M.V. Do microorganisms play a role in irradiation mucositis? Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 861–863.
- Napeñas, J.J.; Brennan, M.T.; Bahrani-Mougeot, F.K.; Fox, P.C.; Lockhart, P.B. Relationship between mucositis and changes in oral microflora during cancer chemotherapy. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2007, 103, 48–59.
- Hensley, M.L.; Hagerty, K.L.; Kewalramani, T.; Green, D.M.; Meropol, N.J.; Wasserman, T.H.; Cohen, G.I.; Emami, B.; Gradishar, W.J.; Mitchell, R.B.; et al. Clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J. Clin. Oncol. 2009, 27, 127–145.
- Al-Ansari, S.; Zecha, J.A.; Barasch, A.; de Lange, J.; Rozema, F.R.; Raber-Durlacher, J.E. Oral mucositis induced by anticancer therapies. Curr. Oral. Health Rep. 2015, 2, 202–211.
- Jensen, S.B.; Jarvis, V.; Zadik, Y.; Barasch, A.; Ariyawardana, A.; Hovan, A.; Yarom, N.; Lalla, R.V.; Bowen, J.; Elad, S. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Systematic review of miscellaneous agents for the management of oral mucositis in cancer patients. Supportive Care Cancer 2013, 21, 3223–3232.
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354.
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461.
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431.
- Chaveli-López, B.; Bagán-Sebastián, J.V. Treatment of oral mucositis due to chemotherapy. J. Clin. Exp. Dent. 2016, 8, e201–e209.
- De Carvalho, P.A.G.; Lessa, R.C.; Carraro, D.M.; Assis Pellizzon, A.C.; Jaguar, G.C.; Alves, F.A. Three photobiomodulation protocols in the prevention/treatment of radiotherapy-induced oral mucositis. Photodiagnosis Photodyn. Ther. 2020, 31, 101906.
- Bensadoun, R.J.; Franquin, J.C.; Ciais, G.; Darcourt, V.; Schubert, M.M.; Viot, M.; Dejou, J.; Tardieu, C.; Benezery, K.; Nguyen, T.D.; et al. Low-energy He/Ne laser in the prevention of radiation-induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Supportive Care Cancer 1999, 7, 244–252.
- Arora, H.; Pai, K.M.; Maiya, A.; Vidyasagar, M.S.; Rajeev, A. Efficacy of He-Ne Laser in the prevention and treatment of radiotherapy-induced oral mucositis in oral cancer patients. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 105, 180–186.e1.
- Zanin, T.; Zanin, F.; Carvalhosa, A.A.; Castro, P.H.; Pacheco, M.T.; Zanin, I.C.; Brugnera, A., Jr. Use of 660-nm diode laser in the prevention and treatment of human oral mucositis induced by radiotherapy and chemotherapy. Photomed. Laser Surg. 2010, 28, 233–237.
- Simões, A.; de Campos, L.; de Souza, D.N.; de Matos, J.A.; Freitas, P.M.; Nicolau, J. Laser phototherapy as topical prophylaxis against radiation-induced xerostomia. Photomed. Laser Surg. 2010, 28, 357–363.
- Gouvêa de Lima, A.; Villar, R.C.; de Castro, G., Jr.; Antequera, R.; Gil, E.; Rosalmeida, M.C.; Federico, M.H.; Snitcovsky, I.M. Oral mucositis prevention by low-level laser therapy in head-and-neck cancer patients undergoing concurrent chemoradiotherapy: A phase III randomised study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 270–275.
- Carvalho, P.A.; Jaguar, G.C.; Pellizzon, A.C.; Prado, J.D.; Lopes, R.N.; Alves, F.A. Evaluation of low-level laser therapy in the prevention and treatment of radiation-induced mucositis: A double-blind randomized study in head and neck cancer patients. Oral Oncol. 2011, 47, 1176–1181.
- Lopes Martins, A.F.; Nogueira, T.E.; Morais, M.O.; de Sousa-Neto, S.S.; Oton-Leite, A.F.; Valadares, M.C.; Aires Freitas, N.M.; Leles, C.R.; Mendonça, E.F. Cost-effectiveness randomized clinical trial on the effect of photobiomodulation therapy for prevention of radiotherapy-induced severe oral mucositis in a Brazilian cancer hospital setting. Supportive Care Cancer 2021, 29, 1245–1256.
- Pires Marques, E.C.; Piccolo Lopes, F.; Nascimento, I.C.; Morelli, J.; Pereira, M.V.; Machado Meiken, V.M.; Pinheiro, S.L. Photobiomodulation and photodynamic therapy for the treatment of oral mucositis in patients with cancer. Photodiagnosis Photodyn. Ther. 2020, 29, 101621.
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Ottaviani, G.; et al. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Systematic review of natural and miscellaneous agents, for the management of oral mucositis in cancer patients and clinical practice guidelines—Part 2: Honey, herbal compounds, saliva stimulants, probiotics, and miscellaneous agents. Supportive Care Cancer 2020, 28, 2457–2472.
- Migliorati, C.A.; Oberle-Edwards, L.; Schubert, M. The role of alternative and natural agents, cryotherapy, and/or laser for management of alimentary mucositis. Supportive Care Cancer 2006, 14, 533–540.
- Baharvand, M.; Jafari, S.; Mortazavi, H. Herbs in Oral Mucositis. J. Clin. Diagn Res. 2017, 11, ZE05–ZE11.
- Aghamohamamdi, A.; Hosseinimehr, S.J. Natural Products for Management of Oral Mucositis Induced by Radiotherapy and Chemotherapy. Integr. Cancer Ther. 2016, 15, 60–68.
- Kaur, M.; Sable, D.M.; Chowdhery, A.; Chavan, M. A Review of Omega 3 and it “s Role in Oral Diseases. Int. J. Curr. Adv. Res. 2016, 4, 921–925.
