Patients with severe COVID-19 infection often develop multi-organ failure. The damage to organs and organ systems is either through direct infection or hampered physiological processes in response to the infection. It is crucial to consider the immune system as the focal point to understand better and integrate the other organs’ complications.
- COVID-19
- stem cells
- blood-brain-barrier
- oxidative stress
- SARS-CoV-2
1. Introduction
The first outbreak of COVID-19 was reported in Wuhan, China, in December 2019. Subsequently, the outbreak spread globally and was declared a pandemic by the World Health Organization in March 2020 [1]. As of October 2021, over 236 million cases have been reported, with 4.8 million deaths worldwide [2]. The mortality rate of COVID-19 is around 2%, and the disease’s spread is exceedingly high [3][4][3,4]. A significant proportion of patients display mild symptoms or are asymptomatic [5]. However, the remainder of the cases are severe, especially in the aged and immune-compromised population [6]. Patients with existing comorbidities such as cardiovascular disease, diabetes mellitus, hypertension, renal dysfunction, liver damage, and cancers exhibit poor prognoses [7]. Death is ultimately caused due to acute respiratory distress syndrome (ARDS), septic shock, cardiac damage, renal dysfunction, and multi-organ failure [8][9][8,9].
The host immune response is thought to play a vital role in the pathogenesis and clinical expression of COVID-19. While immune suppression is a risk factor for infection, the immune system’s hyperactivation in response to infection can cause severe complications and organ damage [10]. Though vaccines are in various development stages, testing, and distribution, there are no robust therapeutics for either treatment or symptomatic management of the disease yet. Another alarming fact is that many recovered patients will suffer from lasting effects and disability due to COVID-19 [11]. Therefore, alternative therapeutic modalities need to be studied and developed for managing severe outcomes of the disease. The US Food and Drug Administration (FDA) is currently exploring various single-agent and combination treatments for the disease. These include antivirals, cell and gene therapies, immunomodulators, and neutralizing antibodies [12].
Stem cell-based regenerative medicine is one such field that might hold the key to reviving tissue damage caused by COVID-19. Stem cells play a significant role in developmental biology because they can differentiate into many cell types. Another advantage is that mesenchymal stem cells (MSCs) exhibit immunomodulatory qualities, regulating the immune responses in patients [13]. There are currently several stem cell-based clinical trials in various stages of development, of which quite a few have shown promise in treating patients [14]. Therefore, it is crucial to understand the molecular mechanisms associated with COVID-19 mediated multi-organ damage to efficiently apply stem cell therapy for its treatment [15].
2. Current Knowledge
The Angiotensin-converting enzyme 2 (ACE2) receptor plays a crucial role in viral entry into the cells [16][17][16,17]. This receptor is found on many cell types present throughout the body, primarily the epithelial cells in the lungs [18] and small intestines [19]. The virus uses its spike proteins to bind to the ACE2 receptor and enter the cell [20]. Once inside a cell, the virus uses the cell’s replication machinery to create several viral copies [21]. These infect neighboring cells leading to loss of physiological function. Once the immune system recognizes an infection, it mounts an immune response to fight that infection. This can lead to inflammation in the infected areas. The immune response usually is well regulated and confined to infected regions [22]. However, in the case of COVID-19, we see a hyperactivation of the immune system, whereby it loses the ability to distinguish between self and foreign, therefore causing damage to healthy uninfected tissue. As damage to the tissue builds up, irreparable damage to the organ can occur, causing the organ to fail. This cascade can occur sequentially or in parallel within other organ systems leading to multi-organ failure and death ( Figure 1 ) [23][24][25][26][23,24,25,26].
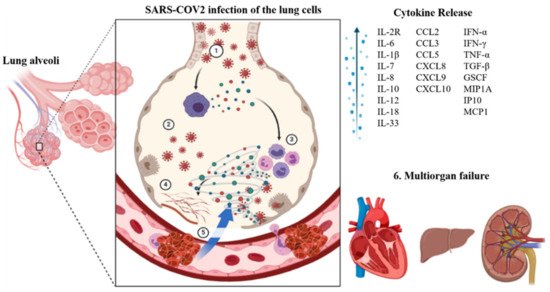
Cytokines are proteins that recruit immune cells to the site of infection. A moderate immune response leads to a rise in proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and interleukin 1 (IL-1), and a host of lymphocytes and T cells. However, in a severe immune reaction, we see a sudden extreme induction of these proinflammatory cytokines, also known as the cytokine storm. Cytokine storms lead to widespread inflammation in the body [27]. As a result, vascular membranes become highly permeable, giving rise to fluid movement from blood vessels into organ tissue [28]. The resulting effect is an organ/tissue reaching the brink of failure due to the lack of blood and oxygen. Anti-inflammatory therapies targeting the cytokine storm are suggested to decrease the mortality of COVID-19 patients. Downstream signaling pathways such as JAK/STAT, NF-κB, and NLRP3 as well as cytokine targets such as IL-6, IL-1 β, IFN-γ, TNF-α, IL-12/23, IL-17A, GM-CSF (granulocyte-macrophage colony-stimulating factor) are being explored for treating COVID-19 induced cytokine storm [29].
Injury to the lungs in COVID-19 may occur through direct or indirect mechanisms. Various cell types in the airways and lungs exhibit the ACE2 receptor. These include type II alveolar cells, ciliated epithelial cells, and pulmonary vascular endothelium. SARS-CoV-2 can infect these cell types and cause cell death directly. Another mechanism involves activating angiotensin-II, resulting in increased vascular leakiness and pulmonary edema leading to pneumonia [30][35]. The immune system also plays a critical role in the clinical manifestation of COVID-19 fibroproliferative lung disease [31][36]. The release of proinflammatory cytokines and activation of macrophages and dendritic cells triggers cell death of infected cells [32][33][37,38]. Another factor responsible for pulmonary failure is microthrombi formation in the vasculature of the lungs [34][39]. Lung biopsies of COVID-19 patients showed an activated complement system in the alveolar epithelial cells with acute and chronic inflammation [33][38].
Patients with cardiac complications caused due to COVID-19 are termed as suffering from Acute COVID-19 Cardiovascular Syndrome (ACovCS) [35][40]. There are two different mechanisms for an individual to develop ACovCS. A hypoxia-induced myocardial injury can occur due to a cytokine storm’s precipitation, as discussed before. This is accompanied by intracellular acidosis and increased oxidative stress. On the other hand, myocarditis can occur through ACE2 facilitated direct infection of cardiac myocytes resulting in arrhythmias or cardiac arrest [36][37][41,42]. However, it is important to note that in a majority of individuals COVID-19 related myocarditis did not accompany immune cell infiltration pointing to cell death from obstructed blood flow likely due to constricted pericytes or clumping of red blood cells [38][43]. The histopathological analysis reported fibrosis and myocyte hypertrophy in most COVID-19 patients [39][44]. Thus, patients with existing cardiovascular disease and hypertension are at heightened risk for mortality due to ischemia and myocardial necrosis factors. Comorbidities such as obesity and diabetes have also been found to indirectly cause adverse cardiac complications. For example, obesity arising out of COVID-19 quarantining is linked with stress, causing a persistent inflammatory state that leads to the deposition of atherosclerotic plaques, rendering obese individuals more susceptible to cardiovascular events [40][41][45,46]. A study reported a greater incidence of diabetes in patients with COVID-19 associated cardiac injury [42][47].
3. Stem Cell Therapy
Stem cells are undifferentiated or partially differentiated cells in the body that can differentiate into various cell types and proliferate indefinitely (self-renewal). Their primary function is to serve as a reserve for the body [43][62]. Stem cells can be of embryonic or adult origin. Based on their functionality, stem cells can be grouped into three categories, pluripotent, hematopoietic, and mesenchymal types [44][45][46][63,64,65]. Pluripotent stem cells can differentiate and mature into any of the three fundamental groups of cells important in human developmental biology. Embryonic stem cells are used for in vitro fertilization purposes [47][66]. Hematopoietic stem cells can differentiate into various types of blood cells. They are obtained from bone marrow or umbilical cord blood and are used in bone marrow transplants. However, both these types of stem cells are currently not used to treat COVID-19. Lastly, MSCs are non-hematopoietic cells that can be differentiated into skeletal tissue such as muscle, bone, cartilage, fat, etc. These cells have immunomodulatory capabilities and have been approved as treatments for a host of autoimmune diseases [48][49][67,68]. Additionally, the therapeutic effects of stem cells were recently ascribed to their ability to replace damaged cells. However, we now know that stem cells’ pro-regenerative quality is also due to paracrine functions and the ability to release microvesicles. Microvesicles are known to contain growth factors, bioactive lipids, anti-apoptotic factors which enhance cell function and stimulate angiogenesis in damaged tissues. They are also known to transfer proteins, mRNA, and microRNA between cells [50][69].
MSCs are referred to as “guardians of inflammation” because of their immunomodulatory effect through the secretion of cytokines, chemokines, growth factors, exosomes, etc. MSCs regulate the inflammatory microenvironment through cell-to-cell contact and the secretion of regulatory molecules. These affect the activation, maturation, proliferation, differentiation, and effector functions of various immune cells involved in innate and adaptive immunity. Innate immunity is mediated through NK cells, macrophages, neutrophils. On the other hand, adaptive immunity is facilitated by T cells and B cells ( Figure 2 ). Dendritic cells (DC) act as the connecting link between innate and adaptive immunity.
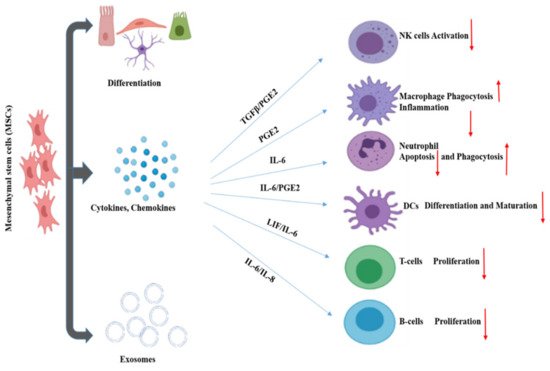
MSCs not only play a role in immune regulation, but also the regeneration and reconstruction of tissue. MSCs have differentiation properties conducive to tissue regeneration and can secrete hepatocyte growth factor, vascular endothelial growth factor, and keratinocyte growth factor. These functions can promote the regeneration of type II alveolar epithelial cells [51][79]. This shows a potential use for MSCs in recovery for severe COVID-19 cases wherein alveolar injury has occurred. It is suggested that MSCs suppress the over-activated inflammatory response, promote recovery of lung function, and potentially influence the progress of pulmonary fibrosis. MSCs have already been shown to significantly contribute to the recovery of patients from severe COVID-19 in Phase 1 clinical trial [14]. A larger phase 2/3 trial is in progress, with 100 participants recruited to evaluate the safety and efficacy of human umbilical cord-derived MSCs as a treatment for severe COVID-19 cases [52][80]. Additionally, MSCs and other stem cell types and derivatives have been indicated to be capable of promoting regeneration in other tissues, such as vascular [53][81], renal [54][82], hepatic [55][83], and neurological [56][84]. Through their immune regulatory functions, and role in contributing to tissue repair MSCs represent a promising area for the treatment of COVID-19 [53][81].
The treatment potential of stem cell therapy has been widely explored in immunological, cardiovascular, renal, pulmonary, and hepatic diseases at the preclinical level. However, the current bulk of clinical data is insufficient to demonstrate the unequivocal efficacy of stem cell therapy in patients with complex diseases like organ failure. Patients with dysfunctional renal and hepatic organs are likely to need organ transplants toward their disease’s end-stage. Kidney failure in chronic kidney disease (CKD) or AKI can be attributed to various complicating factors, including diabetes and heart disease. Similarly, liver failure may be caused by multiple factors such as cirrhosis, Hepatitis B or C, and hemochromatosis, etc. However, there are not enough kidney and liver donors available for the number of needing patients. In this backdrop, stem cell therapy has emerged as a promising option for these patients. Studies have shown minimal adverse events and, in some cases, even positive outcomes for patients undergoing stem cell treatments for liver conditions [57][58][85,86].
4. Conclusions
Patients with severe COVID-19 infection often develop multi-organ failure. The damage to organs and organ systems is either through direct infection or hampered physiological processes in response to the infection. It is crucial to consider the immune system as the focal point to understand better and integrate the other organs’ complications. Given the immunomodulatory properties of stem cells, it is essential to conduct further research to study stem cell therapy’s potential in alleviating COVID-19 multi-organ failure.
