Homocysteine (Hcy) is a non-protein, sulfur-containing amino acid, which is recognized as a possible risk factor for coronary artery and other pathologies when its levels in the blood exceed the normal range of between 5 and 12 μmol/L (hyperhomocysteinemia). At present, standard procedures in laboratory medicine, such as high-performance liquid chromatography (HPLC), are commonly employed for the quantitation of total Hcy (tHcy), i.e., the sum of the protein-bound (oxidized) and free (homocystine plus reduced Hcy) forms, in biological fluids (particularly, serum or plasma). Here, the response of Aerosol Jet-printed organic electrochemical transistors (OECTs), in the presence of either reduced (free) and oxidized Hcy-based solutions, was analyzed. Two different experimental protocols were followed to this end: the former consisting of gold (Au) electrodes’ biothiol-induced thiolation, while the latter simply used bare platinum (Pt) electrodes. Electrochemical impedance spectroscopy (EIS) analysis was performed both to validate the gold thiolation protocol and to gain insights into the reduced Hcy sensing mechanism by the Au-gated OECTs, which provided a final limit of detection (LoD) of 80 nM. For the OECT response based on Platinum gate electrodes, on the other hand, a LoD of 180 nM was found in the presence of albumin-bound Hcy, with this being the most abundant oxidized Hcy-form (i.e., the protein-bound form) in physiological fluids. Despite the lack of any biochemical functionalization supporting the response selectivity, the findings discussed in this work highlight the potential role of OECT in the development of low-cost point-of-care (POC) electronic platforms that are suitable for the evaluation, in humans, of Hcy levels within the physiological range and in cases of hyperhomocysteinemia.
- homocysteine
- organic electrochemical transistors
- point of care testing
- cardiovascular risk
1. Introduction

2. Electrochemical Impedance Spectroscopy (EIS) Experiments on Thiolated Gold SPE

| Fit Parameters | SPE Au Bare | SPE Hcy-Functionalized | ||||
|---|---|---|---|---|---|---|
| R | el | (Ω) | 30.6 | 32.4 | ||
| R | ct | (MΩ) | 1.2 | 4.16 | ||
| CPE (Sxs | n | ) | 5.9 | - | ||
| Ideality factor, n | 0.944 | 0.978 | ||||
| CPE | coverage | (Sxs | n | ) | - | 1.68 |
| R | elpores | (KΩ) | - | 511 | ||
| C | DL | (nF) | - | 330 |
3. Au-Gated OECTs

4. Pt-Gated OECTs
Hcy-based solutions, consisting of Hcy dissolved at various concentrations (from 100 nM to 1 mM) in a physiologic-like microenvironment made of a PBS:BSA (10 mM:600 µM), were used as gate electrolytes for Pt-gated OECTs. Since the neutral electrolytic environment favors the reactivity of the thiol group, oxidative reactions between albumin and Hcy were expected to promote Hcy–BSA binding to a large extent and in a rapid manner [21]. Therefore, the Pt-based OECTs operated in a simil-physiological microenvironment made of some free Hcy and an excess of albumin (BSA–Hcy). The resulting transfer curves are reported in Figure 4a. Interestingly, increasing levels of BSA-Hcy in the electrolytic solution were found to promote an enhanced IDS current modulation.
5. DISCUSSION
In this work, multiple electrical tests (listed in the following Table 2 for the sake of completeness), based on screen-printed electrodes or OECT with different gating conditions, were performed in order to define a suitable experimental strategy to investigate the homocysteine content in different types of solutions.
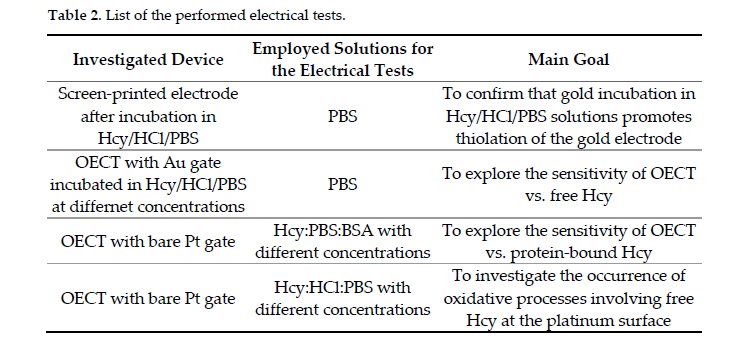
The use of gold or platinum electrodes, in particular, in OECTs allowed the effective detection of Hcy both in the reduced free form (rHcy) and in the presence of albumin-bound Hcy, with this being the most abundant oxidized Hcy-form mimicking a physiological microenvironment.
In the preliminary EIS experiments on thiolated Gold SPE (Section 2.1), the choice of the PBS:HCl solution as the liquid medium for the gold thiolation by free rHcy was justified by the fact that low pH levels generally stabilize against oxidation of this molecule [26]. Given the zwitterionic character of the molecule, the protonation of the amino group at low pH values generates repulsive interactions that limit the oxidative processes of free Hcy molecules. Hence, during the incubation process, the free Hcy concentration in the acidic solution is reasonably maintained around its nominal level, or at least its reduction process is less effective with respect to the case of Hcy in neutral solutions. Nevertheless, it should be noted that the formation of the disulfide occurs through the formation of a thiolate ion [27]. On the other hand, the thiolate ion is probably destabilized by low pH. According our present results, the gold/PBS interface can be modeled using the Randles equivalent circuit (see the inset of Figure 2a), where an electrical resistor, Rel, representing the bulk electrolyte, is in series with a parallel circuit between the Constant Phase Element (CPE) and the charge transfer resistor, Rct. The last parameter takes into account the role of charge transfer mechanisms due to impurities on the (polycrystalline) gold surface [28]. CPEs are commonly used to describe the behavior of real systems and the related impedance can be expressed as ZCPE = 1/(jω)nY0, where n, the ideality factor, ranges between 0 and 1 (for n = 0, it corresponds to the impedance of a resistor, while for n = 1, the CPE corresponds to an ideal capacitor).
As clearly shown in Figure 3a (see the black line), the Randles equivalent circuit adequately fits the behavior of the uncovered (bare) gold surface. Conversely, it completely fails to model the curve achieved for the incubated electrode (Figure S1). In this case, indeed, the gold-Hcy/PBS interface requires an alternative model that is also able to account for the electrode coating. Starting from the circuit model, which is valid for an ideal coverage condition, where Rel is simply in series with a capacitor, real case modifications firstly require the replacement of the capacitor with a CPE, taking into account the surface roughness. Then, it is needed an implementation of the above series in a more complex form, where an element describing the contribution of the uncovered surface (generally pores of different size and surface distribution) is added in parallel to the CPE. This element consists of a series linking the electrolyte resistance through the pores (Relpores, ideally infinite if the coverage is free of pores), as well as a parallel given by the double layer capacitance of the uncovered metal surface (CDL) and a Faradaic impedance (which, in the simplest form, can be a charge transfer resistance). The red line in Figure 3a demonstrates that this model is able to describe the behavior of the incubated surface in a much more convincing way.
Table 1 reports the parameters extracted for the incubated gold surface and indicates that: (i) in this case also, the ideality factor (n = 0.978) value agrees with the formation of a double layer, as expected for non-faradaic systems (which gold/PBS interfaces actually are), which is ascribable to the covered portion of the electrode surface; (ii) the high Relpores value is compatible with the presence of uncovered regions on the electrode surface; (iii) the Rct value is even higher (3.5 times) than that found for the uncovered electrode, suggesting that the coating of a large portion of the surface electrode makes it possible to reduce the impurity-related faradaic contribution.
Figure 3b highlights the difference in total capacitance for Hcy-covered and bare gold electrodes and allows the estimation of the total capacitance at the bare gold electrode (calculated as the intercept value between the real axis and the semicircle [29]), which is about 4 µF. Although such representation is controversial and tricky in terms of estimating the double layer capacitance in cases of defective real coatings described by modified Randles equivalent circuits, the Nyquist plot, reported in Figure 3a for the Hcy-covered Au electrode, qualitatively indicates that its total capacitance is enhanced (i.e., it is expected to be higher than 4 µF).
The experiments with Au-gated OECTs (Section 2.2) show a better response with increasing rHcy concentrations (Figure 4a). This finding may be ascribed to the progressive increase in the electrode capacitance in PBS upon the incubation of the electrode in increasingly highly concentrated Hcy solutions [30]. In addition, the observed enhancement of the gate current values upon incubation of the Au electrode (Figure S3) coherently suggests that the potential drop at the gate electrode/electrolyte interface is actually lowered. In these conditions, a better coupling between the electrolyte and the PEDOT:PSS channel (i.e., larger corresponding gate-induced potential drops) was also obtained.
By calculating the LoD value of our OECT apparatus for rHcy detection; a value of 80 nM was found, with this value being comparable to that of the free-Hcy base level (1–2% of 5 ÷ 15 µM). It is remembering that, even though the acidic conditions were expected to limit the reactivity of the thiol group, it is likely that a free Hcy fraction experienced an auto-oxidation process. This means that the nominal free Hcy concentration value in the considered incubation solutions should represent an upper limit for the real Hcy concentrations. This may happen mostly at the lowest concentrations, where the acidic content of the incubation solution is reduced as a consequence of increasing dilutions of the stock solution in physiological PBS. Finally, our results suggest that a better reproducibility of the sensor response caused by repetitions of the gate voltage sweeps can be achieved by possibly reducing the applied VGS voltage window range. This should be ascribed to the fact that the strength of the SH-Au bond in acidic conditions is not expected to be particularly strong [31] and that the bond may be easily disrupted by repeated measurement.
Furthermore, in the experiment carried out with albumin solution, an increasing level of Hcy promoted an enhanced IDS current modulation (Figure 5a). In this case, however, this finding is basically ascribable to the occurrence of Faradaic reactions at the Pt electrode, which may be mainly related to free Hcy molecules. In fact, it is, for instance, well known that the oxidation of aliphatic amines in platinum can determine the formation of cyanide species which, in turn, can be also adsorbed on the electrode surface, independently of the acidic and basic conditions of their liquid environment [32].
The larger variations of the modulation parameter, in comparison with those achieved for the Au-gated OECT, were due to the higher efficiency of the platinum electrode in sustaining the gate potential via faradaic currents generated upon red-ox reactions at the same electrode. As discussed above, conversely, gold is inert in saline solutions and tends to experience the formation of an EDL, which causes a remarkable gate voltage drop in the electrolyte, i.e., a weaker coupling between the gate electrode and the device channel [33].
The use of Hcy:HCl:PBS solutions as electrolytes (Figure S2a) showed an increase in the LoD (LoD = 2.5 µM) in comparison with the Pt-gated OECT investigated in Hcy:BSA:PBS electrolytic solutions (LoD 180 nM). Since HCl does not significantly affect the OECT modulation in the presence of saline PBS solutions (Figure S4), the observed LoD increase may be explained by invoking the expected protonation of the amine groups, which lowers the concentration of free Hcy molecules and consequently reduces the contribution of Faradaic reactions at the electrode. It should be also remembered that protonation generates repulsive forces between protonated Hcy molecules and the gate electrode (positively biased). As a whole, this is an indirect confirmation of the fact that, in neutral environments (e.g., in Hcy:BSA:PBS), the device response (i.e., IDS modulation) is actually ruled by the oxidative process involving free Hcy at the platinum electrode. In conclusion, the estimated limit of detection (LoD) values is in the range between 80 and 180 nM, and thus, demonstrates the potential of OECT devices for the detection of Hcy in both the physiological range and under hyperhomocysteinemia conditions. Further studies involving specific functionalization protocols for metallic and/or carbon-based gate electrodes will aim to test the response of OECTs in the presence of biological samples in order to confirm their sensibility and to evaluate their selectivity towards a large set of interfering species that are expected to be contained in complex biological matrices.
In conclusion, these results strengthen the potential application of OECT-based methods for the detection of Hcy in biomedicine. Although future work is still required, the approach introduced here could open the way for the future development of POC electronic platforms for Hcy detection, realized on different types of substrates, by relatively low-cost techniques and working at very low voltages (<1 V). The availability of this user-friendly tool could make possible the starting of high-throughput screening campaigns focused on the analysis of Hcy with reference to different pathological conditions. It was pointed out that the determination of tHcy, including all fractions included in Figure 1, is the general strategy for evaluating the levels of this amino acid in biological fluids, as established by the guidelines. Consistently, all current methods used in the clinical laboratory proceed through a reduction step, which basically, lacking selectivity toward individual fractions, allows the detection of all species, at once, as tHcy. However, a number of reports showed that each Hcy fraction has the potential to trigger biological events of specific pathophysiological meaning [34]. For example, protein-bound Hcy was found to exert specific immunological activities [35], while free Hcy was shown to be involved in other mechanisms. OECT, although providing for the determination of tHcy as the sum of individual forms, has the potential to discriminate between protein bound and non-protein bound forms, thus providing additional information of pathophysiological relevance [36–38].
6. MATERIALS AND METHODS
Chemicals
D,L Homocysteine (Hcy), Bovine serum albumin (BSA), Ethylene Glycol (EG), 3-glycidoxypropyltrimethoxysilano (GOPS), H2SO4 and Sylgard 184 polydimethyl siloxane (PDMS) were purchased from Sigma Aldrich (3050 Spruce St Saint Louis,MO., USA) Water dispersion of PEDOT.PSS (Clevios PH1000) was purchased from Heraus (Heraeus Holding GmbH Heraeusstraße 12-14 D-63450 Hanau Germany). Phosphate-buffered saline (PBS) tablets were purchased from Thermo Fisher (Thermo Fisher Scientific, 168 Third Avenue Waltham, MA, USA). Solvents, isopropyl alcohol (IPA), acetone and hydrochloric acid (HCl), were purchased from CARLO ERBA Reagents S.r.l. (Via R. Merendi, 22 - 20007 Cornaredo (MI), Italy)
OECT Fabrication
Gold source and drain contacts were fabricated on a standard thermal SiO2/Silicon wafer using a typical photolithographic procedure described elsewhere [39]. The PEDOT:PSS active layer was deposited using a commercial Aerosol jet Printer (AJP 200, Optomec Inc.- Albuquerque,New Mexico,87109,US-) equipped with an ultrasonic atomizer (UAMax).
PEDOT:PSS dispersion was first sonicated for 30 min, then filtered using a 0.45 mm PES syringe filter (LLG Labware) and mixed with both 5% of Ethylene glycol (acting as secondary dopant) and 1% GOPS (acting as grafting component). The as-prepared solution was diluted in Milli-Q water at a 1:1.5 volume ratio and placed into the ultrasonic vial. The printing process was carried out with a 200 mm nozzle at a speed of 5 mm/s. During this process, sheath gas and carrier gas flows were set 30 and 25 SCCM, respectively, while the plate temperature was set 60 °C [40]. The deposited PEDOT:PSS layer, defining a device channel having width W = 5 mm and channel length L = 200 µm, was thermally annealed at 120 °C for 30 min to improve the related conducting properties. Finally, a PDMS well was stacked over the channel through irradiation of the bottom side of the PDMS well with an UV radiation (NOVASCAN UV-O3 cleaner) for few seconds, in order to promote the reservoir adhesion on SiO2. The final device layout is reported in
Hcy solution Preparation
A stock solution was prepared by dissolving Hcy at 10 mM final concentration in 1 M HCl in PBS. For both gold wire incubation and electrical measurements by bare Pt electrodes, Hcy was diluted in PBS at different concentrations (from 100 nM to 1 mM) and used freshly just after preparation.
SPE Functionalization and Electrochemical Measurements
Commercial SPEs (model 220BT, purchased from Metrohm, (Metrohm Italiana Origgio (VA), Italy), composed of gold working and counter electrodes and an Ag reference electrode, were cleaned with methanol and subsequent cyclic voltammetry (CV) cycles in H2SO4 0.1 M. SPEs were incubated with a single drop (50 µL) of Hcy:HCl:PBS solution (Hcy concentration of 10 mM) for 24 h at 4 °C. Only the working electrode was coated by Hcy solution during the incubation process.
After incubation, electrodes were washed with PBS twice. EIS measurements (Nyquist and complex capacitance plots) were collected in 1x PBS by means of an electrochemical potentiostat/galvanostat (PalmSENS4- Randhoeve 221, 3995 GA Houten, The Netherlands). To this end, an ac probe signal, with amplitude of 10 mV and a sweeping the frequency between 0.1 Hz and 100 KHz, was utilized.
Gold Wire Functionalization and Electrical Measurements.
Gate electrodes were fabricated starting from a % pure gold wire (purchased from VWR), first cut in 5 mm length pieces, then washed in hot acetone and IPA and, finally, immersed in the as-prepared Hcy:HCl:PBS solutions for 24 h at 4 °C to achieve the binding of free Hcy molecules over the gold surface. Finally, the incubated electrodes (Figure 2a) were thoroughly rinsed in PBS and used as gate electrodes in the above described OECT platform.
Transfer characteristics, i.e., channel current (IDS) as a function of the gate voltage (VGS), were recorded accordingly using 2-channel source meter precision unit (B2902A, Keysight Technologies) by fixing the channel voltage (VDS) at −0.1 V and varying the VGS between −0.1 V and 0.8 V (scan rate ΔVGS = 0.02 V; scan time t = 3 s). One batch of PBS was used as a gate electrolyte. After each measurement, the device channel was rinsed three times in MilliQ water, with the aim of removing the residual saline content from the channel and, consequently, restoring the initial IDS current value. The sensing performance was evaluated using the modulation parameter DIDS, defined as [(IDS(@VGS = 0.8) − (IDS(@VGS = −0.1)]/(IDS(@VGS = −0.1). From the DIDS vs. Hcy concentration curve, the Limit of Detection (LoD) was assessed as:
|
LoD = 3σ/S |
(2) |
where σ is the standard deviation of the response (i.e., the standard deviation of the y-intercept of the regression line) and S is the slope of the regression line.
Pt Electrode Electrical Measurements
A Pt wire (purchased from Carlo Erba), 5 mm in length, was cleaned in hot acetone and IPA and used as gate electrode in the 3D-printed OECT platform.
Hcy solutions in the presence of BSA, with a concentration of Hcy ranging from 100 nM to 10 mM, were prepared following the dilution of Hcy in PBS and the subsequent addition of BSA in such a way as to achieve a fixed albumin concentration of 600 µM (physiological concentration). Each of the prepared solutions were used as gate electrolyte in Pt-gated OECT. The electrical characterization was again performed by acquiring transfer curves and extracting the ΔIDS parameter, considering the same biasing conditions and rinsing procedure adopted for the gold-gated OECT.
In a complementary set of experiments, the OECT response in the presence of a bare Pt electrode was assessed using, as a gate electrolyte, some aliquots of the freshly-prepared Hcy:HCl:PBS solutions (with Hcy concentrations ranging between 100 nM and 1 mM) employed for the incubation of the gold electrode. For these measurements, the same previously adopted biasing conditions/rinsing procedures were utilized.
7. REFERENCES
- Nambiar, S.; Yeow, J.T.W. Conductive polymer-based sensors for biomedical applications. Bioelectron. 2011, 26, 1825–1832.
- Khanna, V.K. Electrolyte-gated organic FET (EGOFET) and organic electrochemical FET (OECFET). In Flexible Electronics; IOP IOP Publishing Ltd pp. 8–1 to 8–21; 2019; Volume 2, pp. 2053–2563, ISBN 978-0-7503-2453-3.
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Rev. Mater. 2018, 3.
- Preziosi, V.; Barra, M.; Perazzo, A.; Tarabella, G.; Romeo, A.; Marasso, S.L.; D’Angelo, P.; Iannotta, S.; Cassinese, A.; Guido, S. Monitoring emulsion microstructure by using organic electrochemical transistors. Mater. Chem. C 2017, 5, 2056–2065.
- Jimison, L.H.; Tria, S.A.; Khodagholy, D.; Gurfinkel, M.; Lanzarini, E.; Hama, A.; Malliaras, G.G.; Owens, R.M. Measurement of barrier tissue integrity with an organic electrochemical transistor. Mater. 2012, 24, 5919–5923.
- Van Doremaele, E.R.W.; Gkoupidenis, P.; Van De Burgt, Y. Towards organic neuromorphic devices for adaptive sensing and novel computing paradigms in bioelectronics. Mater. Chem. C 2019, 7, 12754–12760.
- Tarabella, G.; Mahvash Mohammadi, F.; Coppedè, N.; Barbero, F.; Iannotta, S.; Santato, C.; Cicoira, F. New opportunities for organic electronics and bioelectronics: Ions in action. Sci. 2013, 4, 1395.
- Peruzzi, C.; Battistoni, S.; Montesarchio, D.; Cocuzza, M.; Marasso, S.L.; Verna, A.; Pasquardini, L.; Verucchi, R.; Aversa, L.; Erokhin, V.; et al. Interfacing aptamers, nanoparticles and graphene in a hierarchical structure for highly selective detection of biomolecules in OECT devices. Rep. 2021, 11, 9380.
- Preziosi, V.; Tarabella, G.; D’Angelo, P.; Romeo, A.; Barra, M.; Guido, S.; Cassinese, A.; Iannotta, S. Real-time monitoring of self-assembling worm-like micelle formation by organic transistors. RSC Adv. 2015, 5, 16554–16561.
- Gentili, D.; D’Angelo, P.; Militano, F.; Mazzei, R.; Poerio, T.; Brucale, M.; Tarabella, G.; Bonetti, S.; Marasso, S.L.; Cocuzza, M.; et al. Integration of organic electrochemical transistors and immuno-affinity membranes for label-free detection of interleukin-6 in the physiological concentration range through antibody-antigen recognition. Mater. Chem. B 2018, 6, 5400–5406.
- Glushchenko, A.V.; Jacobsen, D.W. Molecular targeting of proteins by L-homocysteine: Mechanistic implications for vascular disease. Antioxidants Redox Signal. 2007, 9, 1883–1898.
- Ueland, P.M.; Refsum, H.; Stabler, S.P.; Malinow, M.R.; Andersson, A.; Allen, R.H. Total homocysteine in plasma or serum: Methods and clinical applications. Chem. 1993, 39, 1764–1779.
- Ingrosso, D.; Cimmino, A.; Perna, A.F.; Masella, L.; De Santo, N.G.; De Bonis, M.L.; Vacca, M.; D’Esposito, M.; D’Urso, M.; Galletti, P.; et al. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet 2003, 361, 1693–1699.
- Maron, B.A.; Loscalzo, J. The treatment of hyperhomocysteinemia. Rev. Med. 2009, 60, 39–54.
- Guthikonda, S.; Haynes, W.G. Homocysteine: Role and implications in atherosclerosis. Atheroscler. Rep. 2006, 8, 100–106.
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. J. 2015, 4. 6. doi: 10.1186/1475-2891-14-6.
- Cheng, M.; Xue, H.; Li, X.; Yan, Q.; Zhu, D.; Wang, Y.; Shi, Y.; Fu, C. Prevalence of hyperhomocysteinemia (HHcy) and its major determinants among hypertensive patients over 35 years of age. J. Clin. Nutr. . https://doi.org/10.1038/s41430-021-00983-6
- Zhao, W.; Gao, F.; Lv, L.; Chen, X. The interaction of hypertension and homocysteine increases the risk of mortality among middle-aged and older population in the United States. Hypertens. . doi: 10.1097/HJH.0000000000003002.
- Rasmussen, K.; Moller, J. Total homocysteine measurement in clinical practice. Clin. Biochem. 2000, 37, 627–648.
- Nekrassova, O.; Lawrence, N.S.; Compton, R.G. Analytical determination of homocysteine: A review. Talanta 2003, 60, 1085–1095.
- Samanta, D.; Sarkar, A. Immobilization of bio-macromolecules on self-assembled monolayers: Methods and sensor applications. Soc. Rev. 2011, 40, 2567–2592.
- Torricelli, F.; Adrahtas, D.Z.; Bao, Z.; et al. Electrolyte-gated trasnistors for enhanced performance bioelectronics. Rev. Methods Primers 2021, 1, 66.
- de Oliveira, G.C.M.; de Carvalho, J.H.S.; Brazaca, L.C.; Vieira, N.C.S.; Janegitz, B.C. Flexible platinum electrodes as electrochemical sensor and immunosensor for Parkinson’s disease biomarkers. Bioelectron. 2020, 152, 112016.
- Adenier, A.; Chehimi, M.M.; Gallardo, I.; Pinson, J.; Vilà, N. Electrochemical oxidation of aliphatic amines and their attachment to carbon and metal surfaces. Langmuir 2004, 20, 8243–8253.
- Mansoor, M.A.; Bergmark, C.; Svardal, A.M.; Lonning, E.; Ueland, P.M. Redox Status and Protein Binding of Plasma Homocysteine and Other Aminothiols in Patients With Early-Onset Peripheral Vascular Disease. Homocysteine and Peripheral Vascular Disease; , 15, 232-240. https://doi.org/10.1161/01.ATV.15.2.232
- Heinecke, J.W. Unique aspects of sulfur chemistry: homocysteine and lipid oxidation. In Homocysteine in Health and Disease; Carmel, R., Jacobsen, D.W., Eds.; Cambridge, United Kingdom, Cambridge University Press, 2001; 31–38.
- Sengupta, S.; Chen, H. Albumin thiolate anion is an intermediate in the formation of albumin-S-S-homocysteine. Biol. Chem. 2001, 276, 30111–30117. : 10.1074/jbc.M104324200.
- McAdams, E.T.; Jossinet, J.; Subramanian, R.; McCauley, R.G.E. Characterization of gold electrodes in phosphate buffered saline solution by impedance and noise measurements for biological applications. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology-Proceedings, New York, NY, USA, 30 August–3 September 2006; pp. 4594–4597.
- Itagaki, M.; Suzuki, S.; Shitanda, I.; Watanabe, K. Electrochemical impedance and complex capacitance to interpret electrochemical capacitor. Electrochemistry 2007, 75, 649–655.
- Nissa, J.; Janson, P.; Berggren, M.; Simon, D.T. The Role of Relative Capacitances in Impedance Sensing with Organic Electrochemical Transistors. Electron. Mater. 2021, 7, 2001173.
- Pacchioni, G. A not-so-strong bond. Rev. Mater. 2019, 4, 226.
- Barnes, K.K.; Mann, C.K. Electrochemical Oxidation of Primary Aliphatic Amines. Org. Chem. 1967, 32, 1474–1479.
- Tarabella, G.; Santato, C.; Yang, S.Y.; Iannotta, S.; Malliaras, G.G.; Cicoira, F. Effect of the gate electrode on the response of organic electrochemical transistors. Phys. Lett. 2010, 97, 123304.
- Jakubowski, H. Homocysteine Modification in Protein Structure/Function and Human Disease. Physiol Rev. 2019, 1, 555-604. doi: 10.1152/physrev.00003.2018.).
- Hoffer, L.J.; Robitaille, L.; Elian, K.M; Bank, I.; Hongsprabhas, P.; Mamer, O.A. Plasma reduced homocysteine concentrations are increased in end-stage renal disease. Kidney Int. 2001, 59, 1, 372–327. doi: 10.1046/j.1523-1755.2001.00500.x.
- Sjöberg, B.; Anderstam, B.; Suliman, M.; Alvestrand, A. Plasma reduced homocysteine and other aminothiol concentrations in patients with CKD. Am J Kidney Dis. 2006, 47, 60–71. doi: 10.1053/j.ajkd.2005.09.032.;.
- Yang, T.H.; Chang, C.Y.; Hu, M.L. Various forms of homocysteine and oxidative status in the plasma of ischemic-stroke patients as compared to healthy controls. Clin Biochem. 2004, 37, 494–499. doi: 10.1016/j.clinbiochem.2004.02.006).
- D’Angelo, P.; Tarabella, G.; Romeo, A.; Marasso, S.L.; Verna, A.; Cocuzza, M.; Peruzzi, C.; Vurro, D.; Iannotta, S. PEDOT:PSS Morphostructure and ion-to-electron transduction and amplification mechanisms in organic electrochemical transistors. Materials 2018, 12, 9.
- Tarabella, G.; Vurro, D.; Lai, S.; D’Angelo, P.; Ascari, L.; Iannotta, S. Aerosol jet printing of PEDOT:PSS for large area flexible electronics. Print. Electron. 2020, 5, 014005.
References
- Nambiar, S.; Yeow, J.T.W. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832.
- Khanna, V.K. Electrolyte-gated organic FET (EGOFET) and organic electrochemical FET (OECFET). In Flexible Electronics; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 2, pp. 8-1–8-21. ISBN 978-0-7503-2453-3.
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086.
- Preziosi, V.; Barra, M.; Perazzo, A.; Tarabella, G.; Romeo, A.; Marasso, S.L.; D’Angelo, P.; Iannotta, S.; Cassinese, A.; Guido, S. Monitoring emulsion microstructure by using organic electrochemical transistors. J. Mater. Chem. C 2017, 5, 2056–2065.
- Jimison, L.H.; Tria, S.A.; Khodagholy, D.; Gurfinkel, M.; Lanzarini, E.; Hama, A.; Malliaras, G.G.; Owens, R.M. Measurement of barrier tissue integrity with an organic electrochemical transistor. Adv. Mater. 2012, 24, 5919–5923.
- Van Doremaele, E.R.W.; Gkoupidenis, P.; Van De Burgt, Y. Towards organic neuromorphic devices for adaptive sensing and novel computing paradigms in bioelectronics. J. Mater. Chem. C 2019, 7, 12754–12760.
- Tarabella, G.; Mahvash Mohammadi, F.; Coppedè, N.; Barbero, F.; Iannotta, S.; Santato, C.; Cicoira, F. New opportunities for organic electronics and bioelectronics: Ions in action. Chem. Sci. 2013, 4, 1395.
- Peruzzi, C.; Battistoni, S.; Montesarchio, D.; Cocuzza, M.; Marasso, S.L.; Verna, A.; Pasquardini, L.; Verucchi, R.; Aversa, L.; Erokhin, V.; et al. Interfacing aptamers, nanoparticles and graphene in a hierarchical structure for highly selective detection of biomolecules in OECT devices. Sci. Rep. 2021, 11, 9380.
- Preziosi, V.; Tarabella, G.; D’Angelo, P.; Romeo, A.; Barra, M.; Guido, S.; Cassinese, A.; Iannotta, S. Real-time monitoring of self-assembling worm-like micelle formation by organic transistors. RSC Adv. 2015, 5, 16554–16561.
- Gentili, D.; D’Angelo, P.; Militano, F.; Mazzei, R.; Poerio, T.; Brucale, M.; Tarabella, G.; Bonetti, S.; Marasso, S.L.; Cocuzza, M.; et al. Integration of organic electrochemical transistors and immuno-affinity membranes for label-free detection of interleukin-6 in the physiological concentration range through antibody-antigen recognition. J. Mater. Chem. B 2018, 6, 5400–5406.
- Glushchenko, A.V.; Jacobsen, D.W. Molecular targeting of proteins by L-homocysteine: Mechanistic implications for vascular disease. Antioxidants Redox Signal. 2007, 9, 1883–1898.
- Ueland, P.M.; Refsum, H.; Stabler, S.P.; Malinow, M.R.; Andersson, A.; Allen, R.H. Total homocysteine in plasma or serum: Methods and clinical applications. Clin. Chem. 1993, 39, 1764–1779.
- Ingrosso, D.; Cimmino, A.; Perna, A.F.; Masella, L.; De Santo, N.G.; De Bonis, M.L.; Vacca, M.; D’Esposito, M.; D’Urso, M.; Galletti, P.; et al. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet 2003, 361, 1693–1699.
- Maron, B.A.; Loscalzo, J. The treatment of hyperhomocysteinemia. Annu. Rev. Med. 2009, 60, 39–54.
- Guthikonda, S.; Haynes, W.G. Homocysteine: Role and implications in atherosclerosis. Curr. Atheroscler. Rep. 2006, 8, 100–106.
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6.
- Cheng, M.; Xue, H.; Li, X.; Yan, Q.; Zhu, D.; Wang, Y.; Shi, Y.; Fu, C. Prevalence of hyperhomocysteinemia (HHcy) and its major determinants among hypertensive patients over 35 years of age. Eur. J. Clin. Nutr. 2021.
- Zhao, W.; Gao, F.; Lv, L.; Chen, X. The interaction of hypertension and homocysteine increases the risk of mortality among middle-aged and older population in the United States. J. Hypertens. 2021.
- Rasmussen, K.; Moller, J. Total homocysteine measurement in clinical practice. Ann. Clin. Biochem. 2000, 37, 627–648.
- Nekrassova, O.; Lawrence, N.S.; Compton, R.G. Analytical determination of homocysteine: A review. Talanta 2003, 60, 1085–1095.
- Mansoor, M.A.; Bergmark, C.; Svardal, A.M.; Lonning, E.; Ueland, P.M. Redox Status and Protein Binding of Plasma Homocysteine and Other Aminothiols in Patients with Early-Onset Peripheral Vascular Disease. Homocysteine Peripher. Vasc. Dis. 1995, 15, 232–240.
