Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Barbara Panunzi and Version 2 by Rita Xu.
The unique role of the zinc (II) cation prompted us to cut a cross-section of the large and complex topic of the stimuli-responsive coordination polymers (CPs). Due to its flexible coordination environment and geometries, easiness of coordination–decoordination equilibria, “optically innocent” ability to “clip” the ligands in emissive architectures, non-toxicity and sustainability, the zinc (II) cation is a good candidate for building supramolecular smart tools.
- zinc (II) coordination polymer
- luminescence
- sensing
1. Introduction
1.1. Brief Historical Background
The work of Alfred Werner (Nobel Prize for Chemistry in 1913) and his contemporaries laid the foundation for the study of coordination polymers (CPs). The first preparation and application of coordination polymers traces back as far as early 18th century, when German chemists accidentally discovered the Prussian blue dye (empirical formula Fe4[Fe(CN)6]3). Crystallographic analysis revealed Prussian blue to be a 3D coordination polymer where Fe2+ cations are coordinated by the carbon atom of the cyanide ligand that acts as a bridge with Fe3+ cations lying in an octahedral N-coordination core. In 1967, J.C. Bailer first introduced the term “coordination polymer”, establishing the constructional requirements and characteristic properties of the CPs as hybrid organic–inorganic materials. More recently, significant advances were due to Professor Richard Robson of the University of Melbourne, who published over 200 articles on coordination polymers and is considered “a pioneer in crystal engineering involving transition metals“ [1][2][1,2]. Specifically, Robson underlined the unicity of the 3D CPs, where the organic ligands extend up to infinity thanks to the coordination bond even in addition to other non-covalent weak interactions. As a pioneer, Robson perceived the structural uniqueness of the regular tridimensional CPs, which today are considered the “frontier” of hybrid responsive materials.
Many time-honoured materials are now recognized as coordination polymers. From the first work published in 1997 [3] to recent years, there are more than 4000 works focusing on specific photophysical properties and/or applications of the coordination polymers (CPs).
1.2. The Role of the Coordination Bond in the Supramolecular Polymers
The polymers are long-chain molecules (macromolecules) built from individual molecular blocks held together via covalent bonds. Where the covalent bond ends, supramolecular materials begin. Supramolecular chemistry concerns non-covalent interactions between small molecules able to self-assemble into macromolecular aggregates. Di-topic or poly-topic ligands with two or more interactive sites can be employed. The supramolecular interactions range from hydrogen-bonding to electrostatic interactions and coordination bonds. The non-covalent “weak” interactions throughout the frameworks direct the self-assembly of mono, bi or three-dimensional (abbreviated as 1D, 2D or 3D) macrostructures [4][5][4,5].
The formation of the coordination bond is the key factor in an eminent number of supramolecular aggregates. In a coordination compound, donor ligands share their electron pairs with a metal atom which is commonly a transition metal cation. Translating this simplified concept to polymers, the coordination polymers (CPs) are solid-state structures consisting of 1, 2 or 3D repeating coordination units arranged in an ordered or not-ordered structural habitus [6][7][8][9][6,7,8,9].
Coordination polymers in which metal ions and organic ligands are linked into 3D structures with a specific nano- (<2 nm) or meso- (2–50 nm) porous structure resulting from a tridimensional crystalline regularity are often referred as metal-organic frameworks (MOFs). Such functional cavities can be target designed for a selective interaction or entrapment, so that MOFs encompass one of the most sought-after class of hybrid responsive materials [10][11][12][13][14][15][10,11,12,13,14,15]. The properties of the supramolecular CPs are based on the association and functionality of the organic building blocks and/or the metal nodes. Several factors play a role towards the engineering of targeted CPs, including association, shape, size, functionality, and connectivity of building blocks. The dimensionality of the CPs depends on the organization of the building blocks, i.e., the organic ligands, the metal ions, the counter ions, and the solvent molecules. The functionality of the ligands is critical for the development of various weak interactions throughout the framework, which in turn are crucial for the construction and spatial arrangement of the material.
An infinite possibility of structures and functions can be designed and realised to direct the interacting sites to target specific applications. In this context, theoretical analysis has constituted a growing support for the design of targeted structures [16][17][18][19][20][21][22][23][24][16,17,18,19,20,21,22,23,24]. The general idea is to exploit the properties of both the organic part and the inorganic part. Metals can conduct electricity and sometimes show magnetic properties. Metals are also catalysts for a wide range of reactions and undergo complexation–decomplexation equilibria under specific conditions. On the other hand, the organic ligands can provide useful optical properties and undergo intermolecular interactions with specific sites and/or analytes. If the sum of properties can be incorporated into a single hybrid material, it could have all sorts of applications. With the feature of high surface area and porosity, tuneable optical properties, adsorption affinity, magnetic properties, and CPs had been utilized in the field of gas storage [25][26][27][28][29][30][31][32][33][34][35][25,26,27,28,29,30,31,32,33,34,35] and separation [36][37][38][36,37,38], chemical sensing [39][40][41][42][43][39,40,41,42,43], energy conversion [44][45][46][44,45,46], heterogeneous catalysis [47][48][49][47,48,49], gas and liquid purification and separation [50][51][52][50,51,52], drugs delivery [53][54][55][56][57][53,54,55,56,57], data storage and processing, and bioimaging [57][58][57,58].
1.3. General Considerations about Chromic Stimuli-Responsive CPs
Stimuli-responsive polymers are often referred to as smart polymers or (generally) functional materials. Their ability to change physical and/or chemical characteristics in response to external stimuli is the result of the specific composition and can be related to de-solvatation, ionization, crosslinking, and finally alteration of the inter-/intra-chains interactions [59][60][61][62][63][64][65][66][67][59,60,61,62,63,64,65,66,67]. It is obvious that CPs, as supramolecular self-assembled polymers, are “par excellence” responsive smart materials [68][69][70][68,69,70].
In such materials, thermodynamics and kinetics play complementary roles. Metal-ligand interactions can be thermodynamically stable but also kinetically labile, depending on the metal ion and ligand. The thermodynamic stability of the metal–ligand interactions will determine the degree of polymerization, the architecture, and the dynamics/lifetime of the coordination aggregate. On the other hand, the kinetic lability should result in a “dynamic” macrostructure in continual equilibrium, therefore, being potentially responsive [71]. CPs can easily respond to an extern stimulus via a dynamic dissociation/recombination equilibrium of non-covalent interactions. Smart functional CPs are attracting a growing interest within the study of life processes and technological applications. Their intrinsic properties make them ideal candidates for analytical and diagnostic dynamic applications. Stimuli-responsive CPs [12][72][73][74][75][12,72,73,74,75] can exhibit changes in a macroscopic property to a specific input, such as light, temperature, physical stress, solvent, analytes, electricity, ionic strength, and pH. The design of tailored stimuli-responsive CPs has attracted significant attention in the past decade due to their stimuli-responsiveness and self-healing ability [76][77][78][79][76,77,78,79].
As a new frontier in the smart materials research, here, we will focus attention to “chromic” materials. By chromic materials, we mean tools able to produce an optical signal within the visible spectrum detectable in absorbance (colorimetric response) and/or in emission (luminescence response) mode, as a response to an external stimulus. Materials able to emit fluorescence in the visible regions, enabling a chromic naked eye response are commonly referred as “fluorophores”. In addition to the obvious advantage to produce a naked eye appreciable signal [80], chromic tools can guarantee a real-time, low expansive, and in situ analysis. The high surface areas, tuneable porosity, and tailorable composition of the coordination polymers perfectly matches the requirements of chromic smart tools. According to the kind of stimulus that they respond to, examples of the major kinds of chromism are:
- -
-
Thermochromism, which is induced by a change of temperature;
- -
-
Mechanochromism, which is induced by a stress in the solid state;
- -
-
Electrochromism, which is induced by the gain and loss of electrons;
- -
-
Solvatochromism, which is induced by the polarity of different solvents;
- -
-
Photochromism, which is induced by light irradiation;
- -
-
Chemochromism, which is induced by specific chemical analytes.
Due to their wide responsiveness, chromic smart materials are now applied as sensors [61][81][82][83][84][61,81,82,83,84], safety devices [85], information and imaging technology [86][87][88][89][90][91][86,87,88,89,90,91], optoelectronic systems [92][93][94][95][92,93,94,95], smart windows and optical switches [96][97][98][96,97,98], inkless and erasable printing [99], and catalysis [100].
The versatile group of CPs provides an infinite number of candidates for the new generation of chromic smart materials, with potential applications both in the bulk state and at the molecular level. Particularly relevant is the photoluminescence (PL) performance which benefits from the tunable hybrid structure and electronic properties of CPs [74]. The general mechanism for luminescence in Cps involves in all cases the absorption of a photon (raising an electron from the ground to an excited state) and the subsequent return to the ground state with the emission of a photon [101]. However, the PL active channels involved in CPs’ emission can be complex and different for each case. Specifically, PL properties can be due to the organic part but also to host–guest interactions or interactions between interpenetrating frameworks. In addition, in CPs, the luminescence often originates from energy bands rather than from discrete energy levels. Finally, different metal cations can bring about radical changes in the PL pattern of the organic ligands. The possible excitations in transition metal complexes will be discussed in Section 1.3, with a specific focus on zinc (II) metal ion.
PL properties may be critical to biological applications. A biosensor must include a biomolecular interaction able to give rise to an output signal. Biology and nanotechnology both inhabit the same length scale, so that nanosized probes are among the recent advanced tools for sensing of biological relevant entities and/or parameters in medical diagnostic. On the other hand, when materials are reduced to the nanoscale (5–200 nm), their properties can be dramatically altered respect to those observed in bulk. Miniaturization of CPs to the nanoscale offers a unique opportunity to assemble highly customizable functional materials. Responsive nanoscaled coordination polymers (NCPs) marry the tunability of the coordination complexes to the advantages of nanoprobes for an optimal use in sensing, recognition, and diagnosis processes. Specifically, MOF probes with nanosized cavities can meet the intrinsic theranostic properties of specific organic ligands with the biocompatibility and/or low cytotoxicity of the metal. Up to now, they have a unique impact in prospective of drug encapsulation and delivery.
As will be discussed, the tuneable and tailoring absorption and emission properties of the zinc-based CPs make them ideal candidates as both colorimetric and fluorophore smart materials. In the next section the unique role of the zinc (II) cation in the backdrop of the stimuli-responsive CPs will be underlined and discussed.
1.4. The Charming Peculiarity of Zinc Cation
The self-assembly of polydentate ligands and transition metal cations provides endless architectures and infinite behaviours. The construction of CPs with tailored properties and targeted applications is a seething research area. The interest is driven by the impact on the technology and the economy. Technicians and users are addressing the demand to the challenge of sustainability. Alternative cheap “green matter” is gaining traction over toxic and expensive products. The use of zinc cation in building of supramolecular CPs moves in this direction.
From a structural point of view, the d10 closed-shell metal ion Zn2+ is suited for the construction of varied coordination architectures [102]. Zinc (II) cation can adopt three coordination numbers, i.e., 4, 5, and 6. The energetic cost for hydrated ion to switch among the three coordination numbers is within 0.4 kcal mol−1 in the gaseous phase. Such ability contributes to the effectiveness of Zn (II) as a catalytic centre easily undergoing coordination–decoordination equilibria depending on the environmental conditions [103]. Due to its flexible coordination environment and geometries, zinc ion can accommodate different structural arrangements such as linear or zigzag chains, ladder, square, rhombic, brick-wall structures, and diamond networks (Scheme 1) [22].
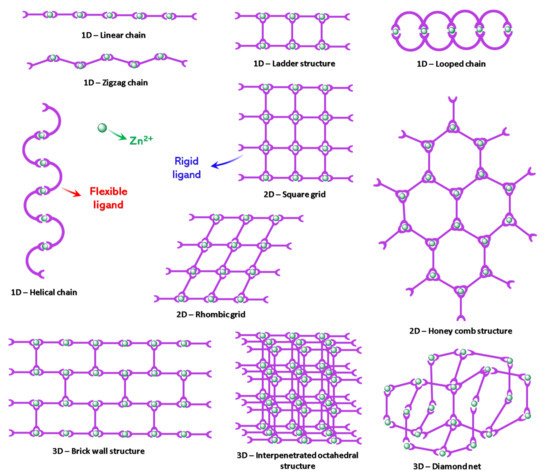
Scheme 1.
Relevant topological types of Zn-CPs.
Starting from the mono and bi-dimensional macro-sized CPs employed in sensing of metals, small organic molecules, and environmental pollutants to the highly engineered three-dimensional nano-architectures able to cell permeation, zinc (II) cation can realise limitless combinations of 1D, 2D and 3D polymeric structures [55][56][57][58][55,56,57,58]. Zinc-based MOFs and nanoprobes are now a significant group of versatile engineered tools for medical diagnostic. They are currently employed for the detection of bioanalytes, as drug carriers and as chemotherapeutics, and to monitor and/or mark specific cells and subcellular compartments and parameters. Both as macro and nano-sized tools, chromic zinc-based CPs can be rightly placed among the emerging fluorophores used as smart probes.
Luminescence in CPs (LCPs) arises from the characteristic of the organic ligands which are coordinated to the metal centres or clusters [43][104][43,104]. Guest molecules get entangled into the frameworks leading to the emission response (enhancement or quenching of luminescence). From a theoretical point of view, the origin of the luminescence processes in the hybrid polymers arises from one or several of the following electron transfer mechanisms: ligand cantered (LC) luminescence; metal cantered (MC) luminescence; luminescence arising from charge transfer (CT) states, which includes ligand-to-ligand (LLCT), ligand-to-metal (LMCT), and metal-to-ligand (MLCT) charge transfers.
2. Zn-CPs Responsive toward Metal Cations, Oxyanions, and Small Molecules
The detection principle in sensing by CPs is the interaction of the probe with molecules/ions due to potential interacting sites present at the ligand or the metal. The interaction can alter the structural pattern and/or the orbital energy levels of the material. Specifically, in chromogenic LCPs, the emission position and/or intensity can be altered by the presence of an analyte able to change the ground- and/or the excited-state of the sensing probe [43][104][43,104]. In this section, particular attention has been paid to the luminescence properties of Zn-LCPs and their applications as chemical sensors.
Zn-CPs offer a suitable platform for a novel generation of colorimetric and/or luminescent smart sensors. The role of the zinc (II) ion is crucial in the turn-on/turn-off chromic response of the sensing material. Thanks to the varied structural ability and to the coordination–decoordination equilibria of the zinc (II) coordination core, limitless polymeric architectures can be dissolved and reformulated [105][106][126,127]. The interactions between ligands and zinc nodes can be destroyed and recovered by adding competitive analytes, resulting in a change of the natural colour or in a fluorescence quenching or enhancement, due to structural and/or electronic rearrangements. In some cases, also the dimensionality of the CP matrix can undergo a substantial change, turning 1D and/or 2D polymers into 3D networks. Finally, the sought-after selectivity and real-time response properties are often guaranteed by Zn-CPs probes.
In the last five years, Zn-CPs were successfully employed in the sensing of metals, small organic molecules, and ions [107][108][109][110][111][128,129,130,131,132]. The sensing ability toward metals is a relevant property. Metals are essential to chemical, enzymatic, and biological processes; on the other hand, they may represent environmental pollutants. Other hazardous pollutants are small molecules/ions such as chromates and dichromates, azo compounds, nitroaromatic compounds, and volatile organic compounds (VOCs). Trapping and detection of such chemicals is a hot topic in the field of stimuli-responsive probes. In this section, some relevant articles will be summarized highlighting and discussing the relationship between structure (derived by X-ray diffraction analysis), dimensionality, and sensing ability.
Due to their unique crystalline structure and voids, high surface area, and functionalities, regular 3D CPs (mainly MOFs) are the ideal versatile candidates for selective sensing [112][113][114][115][133,134,135,136]. In designing a responsive luminescent MOFs (LMOFs), the electronic structure of the host frames and the host–guest interactions must be considered. The geometry of the voids and the interacting binding site lead to selective sensing properties in a single or multi-functional mode. Obviously, the nature of the metal ion affects the structural pattern, the chemo- and physical properties, and finally the sensing specificity and mechanism. Both a fluorescence enhancement or quenching can be the result of the interaction between the MOF cavities and the analyte, due to the molecular and electronic changes of the guest–host system. If the target analyte interferes with the ligand’s electronic structure reducing the energy transfer from the ligand to the metal, the sensing process results in a decrease of the fluorescence intensity. But if the analyte moves the activation of a novel emissive channel, a fluorescence enhancement can be obtained. Zn-LMOFs typically emerge as strong luminescent probes due to the CHEF effect imposed by zinc (II) ion. They can both retain or quench the fluorescence response of the linkers mainly related to LCT and/or LLCT transitions.
2.1. Mono or Multifunctional Metal Cations Sensing
Recent relevant examples of Zn-CPs metal sensing were presented in 2017–2019. In 2017 Jian-Ping Lang and coworkers [107][128] presented a deeply characterised series of a 1D and four 3D Zn-CPs obtained by ligands 1,4- bis(2-(pyridin-4-yl)vinyl)naphthalene and 1,3,5-benzene-tricarboxylic acid in different molar ratio. The structural pattern of the five CPs was explored, and their luminescent response toward Hg2+ was examined. Specifically, the more representative example resulted highly selective and sensitive for the detection of Hg2+ ion. A finely ground sample suspended in N, N dimethylformamide (DMF) solvent undergoes a fluorescence quenching and a change of fluorescence colour from blue to yellow naked eye perceivable under UV light. As an essential trace element in the human body, crucial in several biological processes, iron ion is a desirable metal target. In 2020, Zhong-Feng Shi and coworkers [116][137] published another prominent article on two Zn-CPs produced by hydrothermal reactions of ligands 5-sulfosalicylic acid and 1,4-bis(1H-imidazol-1-yl) benzene (1,4-bib) for different reaction time. After two days reacting, a 2D structure was obtained, while a 3D coordination polymer was obtained after the same mixture reacted for three days, each structural unit being linked by ten 1,4-bib ligands to form a 3D MOF. The 2D CP shows excellent fluorescence quenching response toward Fe3+ and the 3D CP toward Hg2+. The quenching mechanism was explored in both cases and ascribed to the absorption of the emitted light for Fe3+ and to the electron transfer and weak interaction of Hg2+. In 2018, two Fe (III) quenching responsive and highly selective Zn-MOFs were described. Specifically, Huan Yang and coworkers [117][138] (see Figure 1) produced a luminescent Tb3+ and Eu3+ encapsulated Zn-MOF based on 5-(5-norbonene-2,3-dicarboximide)isophthalic acid, and Guangshan Zhu and coworkers (see Figure 1) [118][139] obtained a mixed Li+ and Zn2+ based MOF from the quadridentate carboxylate ligand 5-(bis(4-carboxybenzyl)amino)isophthalic acid.
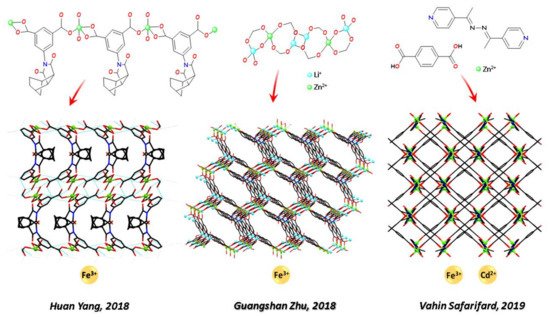

The simultaneous detection of selectively trapped metal cation is a desirable property of some LMOFs. Many Zn-MOFs for simultaneous detection of iron and another metal cations are known, such as the Eu3+ encapsulated Zn-LMOF based on tetrakis(4-pyridyloxymethylene)methane and 2,6-naphthalenedicarboxylic acid able to detect Fe3+ and Cr4+ ions (Xin Liu and coworkers [120][140], 2017); the Zn-MOF responsive to Fe3+ and Al3+ ions (Xia Li and coworkers [121][141], 2018) assembled from 3,3′-diphenyldicarboxylate and 1,4-bis(1,2,4-triazol-1-yl)butane; the Zn-MOF named TMU-16 from a bipyridyl ligand with a bridging azine group (Vahid Safarifard and coworkers, 2019, see Figure 1, [122][142],) displaying a luminescence quenching response to Fe3+ and a luminescence enhancement response to Cd2+ ions.
2.2. Multi-Sensing of Metal Cations, Oxyanions, and Nitro Aromatic Compounds
Among the innumerable LCPs, several Zn-LCPs have drawn attention as highly selective multi-analytes probes. Simultaneous operativity is a sought-after property, and it enables the probe to be employed in an actual technological use. Multi-responsive Zn-CPs explored in the last years can detect simultaneously metal cations (mainly Fe3+), anions, and small molecules. Specifically, common carcinogenic pollutants of aqueous solutions used in several industrial applications [123][124][125][143,144,145] such as NACs (nitro aromatic compounds) and chromium (VI) oxoanions (Cr2O72−, CrO42−) are the target analytes of 3D Zn-CPs.
Recent examples are the work of Bao-Long Li and coworkers, which in 2018 [126][146] studied the crystalline pattern of five Zn-CPs based on the ligand 1,3-bis(1,2,4-triazol-4-ylmethyl)benzene with different co-ligands (btec = 1,2,4,5-benzenetetracarboxylate, Meip = 5-methylisophthalate, nip = 5- nitroisophthalate, hip = 5-hydroxyisophthalate, nbdc = 4-nitro-1,2-benzenedicarboxylate). The CPs display different structural patterns and dimensionality from 1D to 3D. The 3D CP shows a (4,4)-connected self-catenated 3D network of the 4,4T32 topological type and is a highly sensitive and selective luminescence sensor for Fe3+, Cr2O72−, and CrO42− in aqueous solution, with detection limits of 6.28, 3.05, and 5.72 μM, respectively, and an assumed quenching mechanism. In the same year, Lin Dua, Qi-Hua Zhao, and coworkers (see Figure 2) [127][147] synthetised three novel LCPs from the flexible zwitterionic ligands 1,1′,1″-(2,4,6-trimethylbenzene-1,3,5-triyl)-tris(methylene)tris-(4-carboxypyridinium)tribromide and 1,2,4,5-tetrakis-(4-carboxylatopyridinium-1-methylene)benzene and zinc (II) or cadmium (II) d10 cations. They compared the different structural pattern and dimensionality promoted by the metal and the related sensing ability toward Fe3+, nitrobenzene (NB), and HSO4− anions.
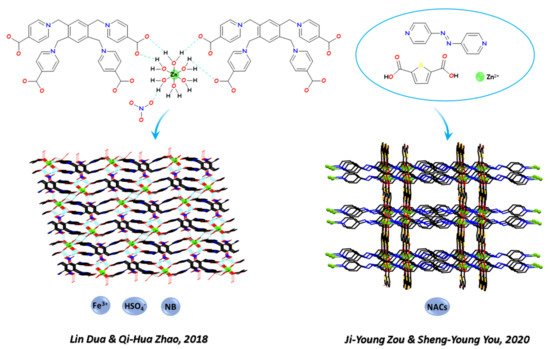

In 2019, Hao Lei and coworkers [128][148] obtained a series of 2D Zn-CPs by ionothermal reaction a series of 2D Zn-CPs named {Zn2 × 2(BDC)(L)}n where X = Cl (1 and 3) or Br (2 and 4), BDC = 1,4-benzenedicarboxylate, L = 4,4′-bipyridine (4,4′-bpy, 1 and 2) or 1,4-diazabicyclo [2.2.2]octane (dabco, 3 and 4). The polymer 1 (with chloride and 4,4′-bpy) displays strong luminescence in the solid state and selective luminescence quenching for Fe3+ ions and nitroaromatic compounds (NACs) in ethanol solutions, while the polymer 4 (with bromide and dabco) can selectively adsorb Congo red dye from other dye molecules. In 2020, Yujuan Zhang and coworkers [129][149] used copper (II) in a case and zinc (II) in another case as metal nodes to produce two CPs, namely [(Cu(bib)2)⋅2NO3⋅H2O]n and [[Zn3(bib)2.5(C2O4)3(H2O)]] DMF⋅H2O]n. By structural analysis, the polymeric arrangements were compared and both CPs were found to be 2D frameworks further expanded into 3D supramolecular structures through C–H⋯O hydrogen bonding. The sensing pathway was examined. The CPs were found to be quenching responsive to Fe3+ and NACs, through a PET (photoinduced electron transfer) and RET (resonance energy transfer) mechanism for NACs, and a RET mechanism for Fe3+, respectively. Very recently, Baokuan Chen and coworkers [130][150] explored the influence of carboxylic acid substituents on the structural and emissive pattern of three Zn-CPs built from isophthalic acid (1), 5-hydroxyisophthalic acid (2), and 5-nitroisophthalic acid (3), respectively, and the N-donor ligand N,N’-bis(4-methylenepyridin-4-yl)-1,4-naphthalene dicarboxamide. The CPs have similar 4,4-connected 2D structures exhibiting different structural details and show multi-functional fluorescence response towards metal ion (Fe3+), anions (MnO4−, Cr2O72−, CrO42−), and 2,6-dichloro-4-nitroaniline. The luminescence quenching effect was not ascribed to any skeleton collapse but to an energy competitive absorption mechanism, which leads to a RET effect.
Zn-CPs can be useful tools also for detection of rare heart metals. Jianing Xu and coworkers (see Figure 3) [131][151] in 2017 obtained two Zn-LCPs based on ligand 1- (triazol-1-yl)-2,4,6-benzene tricarboxylic acid and 1,2,4-triazole (1) and 1,10-phenanthroline (2), respectively. CP1 shows a 3D framework helical chain and CP2 shows a 2D layered network extending to 3D structure through π–π stacking interactions. Both CPs perform as hosts for the encapsulation of Ln3+ ions and serve as antennae to sensitize Tb3+ ions. In addition, CP2 exhibits highly luminescent sensing properties for acetone.
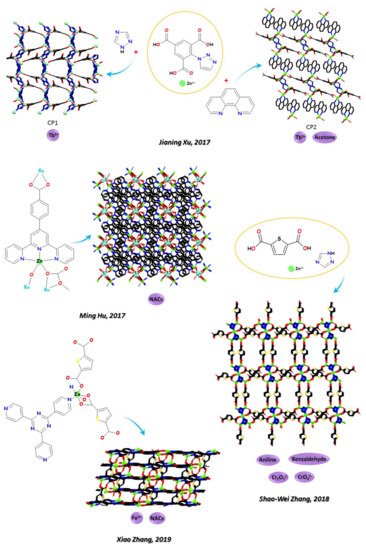

Recently, examples of 3D tools as Zn-LMOFs selectively detecting nitro derivatives were studied in depth both from the structural and functional point of view. In 2020, Ji-Young Zou, Sheng-Young You [132][152], and coworkers (see Figure 2); in 2017, Ming Hu and coworkers (see Figure 3) [133][153]; and in 2019, Xiangyang Qin and coworkers [134][154] ascertained the quenching mechanism imposed by the presence of NACs in a series of Zn-LMOFs where a ruling FRET process affected the luminescence response of the probe. In other cases, multifunctional Zn-LMOFs was able to selectively detect NACs and/or Cr2O72− and CrO42− anions, and/or Fe3+, and a series of selected examples are briefly reported below. As relevant examples, in the same year (2018), Suna Wang and coworkers [135][155] produced two Zn-LMOFs based on 2,2′-[benzene-1,3-diylbis(methanediylsulfanediyl)]dibenzoic acid, 1,3-bis(4-pyridyl)propane, 1,2-Bis(4-pyridyl)ethylene, and DMF (N,N-Dimethylformamide), and He-Gen Zheng and coworkers [136][156] produced three Zn-LMOFs, based on E,E-2,5-dihexyloxy-1,4-bis-(2-pyridin-vinyl)-benzene, 1,4-cyclohexanedicarboxylic acid, 4,4′-oxybisbenzoic acid, and 4,4′-sulfonyldibenzoic acid for selective detection of Fe3+ and Cr2O72-. The same couple of analytes were selectively detected by the MOF named [Zn(dptz)(BDC)(H2O)]n [1, dptz = 3,6-di(1H-pyrazol-4-yl)-1,2,4,5-tetrazine, H2BDC = terephthalic acid] (Xian-He Bu and coworkers [137][157], 2020) and by the MOFs named [Zn2(HL) (phen)]n and [Zn2(HL)(2,2-bipy)]n obtained from the V-pattern multi-carboxylic acid ligand H5L = 3,5-di(2′,5′-dicarboxylphenyl)benzoic acid (Tuoping Hu and coworkers 2019 [138][158]).
NACs and Fe3+ can be selectively detected by a Zn MOF with a 3-fold interpenetrating 3D framework based on tpt = 2,4,6-tri(pyridin-4-yl)-1,3,5-triazine and H2tda = 2,5-thiophene dicarboxylic acid (Xiao Zhang and co-workers in 2019, see Figure 3 [139][159]) and by the MOFs obtained by 4,40,400-nitrilotribenzoic acid (H3NTB) and 1,10-phenanthroline (phen) (Lirong Yang and coworkers in 2021 [140][160]). In all cases, a quenching response was detected for both analytes. Multi-responsive probes for NACs, Fe3+, and Cr (VI) oxo-anions were designed by Zhong-Feng Shi and coworkers in 2020 [141][161] based on 3-nitro-4,4′-biphenyl dicarboxylic acid and 11,4-bis(imidazole-1-ylmethyl)-benzene. Aniline, benzaldehyde in DMF and Cr2O72−/CrO42− anions in water can be selectively detected by the Zn-MOF based on a pentametallic clusters and thiophene-2,5-dicarboxylic acid (Shao-Wei Zhang and coworkers (see Figure 3) [142][162], 2018).
2.3. Multi-Sensing of Biologically Harmful Small Organic Molecules
Many 3D Zn-CPs have been designed to be highly selective toward specific small molecules. Zn- MOFs responsive towards environmental organic pollutants, such as VOCs and water-soluble organic dyes and pesticides, are highly required in water quality tests. In many cases, they are selective multifunctional probes. Very recently, a variety of Zn-LMOFs probes selectively responsive towards biologically harmful molecules generated as wastewater from industrial processes [143][163] have been produced.
Huai-Ming Hu in 2016 [144][164] prepared three Zn-CPs based on 40-(4-carboxyphenyl)-60-carboxycalte-2,20-bipyridine and glutaric acid. Depending on different pH values and auxiliary ligands, different 2D (hcb topological net and layer structure) or 3D (eight-membered rings self-penetrating MOF structure) CPs were obtained. The Zn-LMOF shows high-sensitivity sensing to metal cations (Fe3+ and Cu2+) and harmful small organic molecules (as methanol and nitrobenzene). More recently, in 2020, Jianrong Li [145][165] and coworkers synthetised a 3D Zn-LCP by the reaction of Zn(NO3)2, N,N′- bis (3-pyridinecarboxamide)-1,4-butane and 4, 4′- oxidiphthalic acid. Besides the in-deep structural analysis, the polymer demonstrated remarkable fluorescent properties and chemical stability under an acidic or alkaline environment. It was used as a multifunctional chemosensor for detection of metal cations (Fe3+, Bi3+), oxyanions (MnO4−, Cr2O72−), and toxic organic solvents such as NB, acetaldehyde, and acetylacetone. The mechanism of sensing process indicated that the synergistic effect of electron transfer by both luminescent ligands and the FRET mechanism leads to fluorescence quenching.
In some cases, Zn-LMOFs are designed to be highly selective toward highly specific small molecules. In 2018, Jian-Zhong Cui and coworkers [146][166] produced an anionic Zn-MOF from furan-2,5-dicarboxylic acid and 1H-benzotriazole working as a turn-off luminescent sensor toward a volatile and flammable analytical reagent, acetylacetone. The selective structural cavities of MOFs make them ideal candidates for detecting VOCs upon adsorption. Patima Nizamidin and coworkers in 2019 [147][167] proposed a series of Zn MOFs, derived from terephthalic acid and N,N’-di(4-pyridyl)-1,4,5,8-naphthalenediimide. Formed in thin glassy films, the MOFs membranes display a selective adsorption response to meta-xylene gas. In 2019, Bing-Hui Wanga and Bing Yan [148][168] designed a Zn-MOF based on a fluorescein dianion functionalized dye for the detection of the small trichloroacetic acid (TCA), a carcinogen metabolite in human urine.
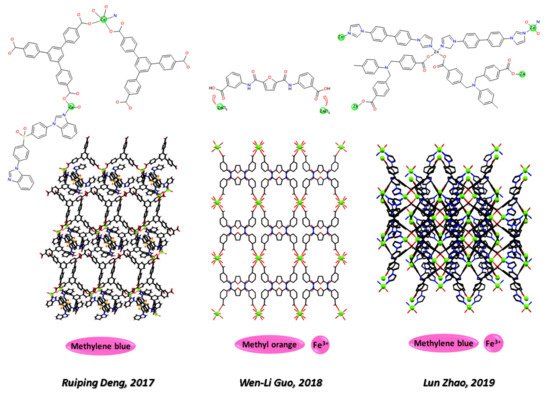
Zn-MOFs responsive towards environmental organic pollutants, such as organic dyes and pesticides, are sensing tools in water quality tests. In 2017, Ruiping Deng and coworkers (see Figure 4) [149][169] reported a Zn-MOF built from bis(4-benzylimidazol-ylphenyl)sulfone and 4,4′,4′’-benzene-1,3,5-triyltribenzoic acid responsive to methylene blue. Wen-Li Guo and coworkers (see Figure 4) in 2018 [150][170] and Lun Zhao and coworkers (see Figure 4) in 2019 [151][171] studied two multifunctional systems for sensing of iron and a dye. Based on an 8-fold interpenetrating diamond network of N,N0-bis(4-carbozylbenzyl)-4-aminotoluene) ligand [151][171] and on one of bis-(3-carboxy-phenyl)furan-2,5-dicarboxamide [150][170], respectively, the first one selectively traps methylene blue and the second one methyl orange dye.

In 2020, three relevant articles were produced on multifunctional MOFs probes for environmental pollutants. Jarugu Narasimha Moorthy and coworkers [152][172] synthetised a Zn-MOF with ca. 27% solvent-accessible void volume based on a tetracarboxylic acid ligand with a twisted dibenzo[g,p]chrysene core (2,7,10,15-tetrakis [2,6-dimethyl-4-(α-carboxy)methoxyphenyl]-dibenzo[g,p]chrysene) highly specific in water toward hazardous “quat” dicationic herbicide with diquaternary bipyridyl motifs. Xiutang Zhang and coworkers [153][173] used a 3D self-penetrated framework based on 1,3-bis (imidazol-1-ylmethyl)benzene and π-conjugated aromatic p-terphenyl-2,2″,5″,5‴-tetracarboxylate acid for DCN (2,6- dichloro-4-nitroaniline) pesticide and for nitrofuran NFT (nitrofurantoin) and NTZ (nitazoxanide) antibiotics. Finally, Qinhe Pan and coworkers [154][174] designed a Zn MOF for the detection of U(VI) as a potential environmental pollutant.
