The membranous extracellular matrix (ECM) decellularized from body membranes (e.g., pleura, peritoneum, and amniotic membrane, etc) retain multiple bioactive components like collagen, laminin, fibronectin, growth factors, and polysaccharide. In addition, they also possess ultrastructure features similar to that of the natural tissues with distinct advantage in high density cell seeding, the migration of repair cells from adjacent tissues, and the mass exchange between tissues. These merits make membranous ECM scaffolds extremely attractive in regenerative medicine including but not limited to skin wound healing.
1. Introduction
Body membranes are thin layers of cells or tissues covering the surface of body, the internal organs, or the body cavities. Generally, they can be classified into two categories: (1) the epithelial membranes, and (2) the connective tissue membranes [1][4]. The epithelial membranes, which are composed of epithelial tissue and fibrous connective tissue, can be further divided into (1) the cutaneous membrane (i.e., skin), (2) the serous membranes, such as pleura, peritoneum, and amniotic membrane, and (3) the mucous membranes [1][4]. Unlike epithelial membranes, connective tissue membranes (e.g., periosteum, fascia, and synovial membrane) are typically composed of cells, ground substance, and connective tissue fibers [1][4].
After decellularization, extracellular matrix (ECM) scaffolds obtained from different types of body membranes retain a variety of bioactive substances such as the growth factors, collagen, laminin, fibronectin, and polysaccharide. Notably, they possess ultrastructure features similar to that of the natural tissues [2][3][4][5,6,7]. These microporous thin films have a distinct advantage in the mass exchange between tissues [5][6][8,9]. Particularly, scaffolds in a thin planar form are favorable for high density cell seeding and the migration of repair cells from adjacent tissues [5][7][8,10]. These merits make membranous ECM scaffolds extremely attractive for skin wound healing.
Indeed, the safety and efficiency of several membranous ECM scaffolds have been verified in many clinical practices; however, some of their physicochemical properties, such as the mechanical strength and the degradation characteristics, are far from satisfactory for broad applications. This is partially due to the damage of the crosslinked networks of natural tissues during scaffold preparation, especially the use of acids, alkalis or proteases for decellularization. It is well-known that an ideal scaffold for tissue repair should possess good biocompatibility, robust bioactivity, suitable degradation, and proper mechanical properties. Therefore, attempts to endow traditional membranous ECM scaffolds with desired properties have been the focus of many researches. For instance, to meliorate the physical or chemical defects of traditional ECM scaffolds, different crosslinking methods have been developed [8][11]. Furthermore, diverse macromolecules, either natural or synthetic, have been used as functional additives to produce ECM-based implants [9][10][11][12][13][12,13,14,15,16]. In light of the functional requirements of normal wound healing, many types of biomolecules, nanoparticles, and drugs have been utilized to engineer new generations of ECM-based biomaterials, which can stimulate a specific wound healing stage or event to facilitate chronic wound healing.
2. Traditional ECM Scaffolds Derived from Body Membranes for Skin Wound Healing
Traditional ECM membranes derived from human or animal tissues, such as pericardium, peritoneum, and chorion, have been utilized to facilitate skin wound healing
[14][15][16][17][18][19][17,18,19,20,21,22]. Among them, acellular dermal matrix (ADM), small intestinal submucosa (SIS), and acellular amniotic membrane (AAM) are representative biomaterials that have been commercialized and extensively applied in the clinic
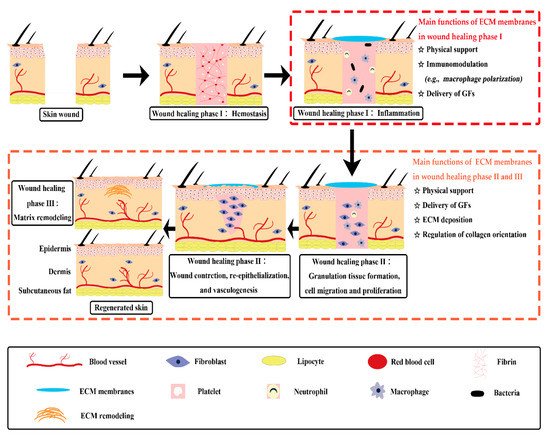
[18][20][21][21,23,24]. The exact wound repair mechanism of membranous ECM scaffolds in living organisms remains to be fully understood. But it has been assessed that, besides physical support, traditional ECM membranes have functions of immunomodulation, growth factor stimulation and ECM regulation, which can trigger several crucial events during wound healing process (
Figure 1)
[7][10]. Chronic wounds usually experience a prolonged inflammation phase with some abnormal healing events. Some ECM membranes, like SIS and acellular pericardium, were proved to have immunomodulatory ability. They are capable of triggering the macrophages to express a predominant M2-like phenotype, which can secret pro-healing cytokines to initiate the anti-inflammatory and pro-remodeling process
[22][23][25,26]. Moreover, some ECM components possess bioactive motifs to regulate cell adhesion and proliferation, such as the Arg–Gly–Asp (RGD) motif. The special domain of RGD peptide is capable of converting the inflammatory response towards a pro-healing response through the binding with integrins of macrophages to modulate signaling pathways involved in cell migration, adhesion, and inflammatory activation
[24][27]. Besides, the inherent growth factors of ECM membranes may provide a complex signaling milieu to stimulate granulation tissue formation, moderate cell transition, angiogenesis, and matrix formation and remodeling during the wound healing phases
[25][26][28,29].
Figure 1. Diagram depicting concept and mechanisms of skin wound healing assisted by traditional ECM membranes. ECM membranes have multiple functions in the different phases of cutaneous wound healing. ECM: extracellular matrix; GFs: growth factors.
2.1. Acellular Dermal Matrix (ADM)
Produced from human or animal skin, ADM is favorable for full-thickness skin wound healing and can reduce scar tissue formation
[18][27][28][29][21,30,31,32]. After transplantation in the wound bed, ADM enhances the synthesis of hyaluronic acid and induces wound angiogenesis
[26][30][31][29,33,34]. Currently, there are several ADM products from human skin, such as the AlloDermTM, GraftJacket
®, and SureDerm
® [32][35]. AlloDerm™ has been utilized to cover deep burn wounds in a case series. The application of AlloDerm™ resulted in excellent graft take, good elasticity, little contracture, and few scarring
[33][36]. GraftJacket
® has been recommended in the treatment of diabetic wounds or the replacement of damaged or inadequate integumental tissue
[34][37]. When compared with standard wound care, it was reported that a single application of GraftJacket
® can reduce the mean wound healing time of diabetic foot ulcers
[35][38].
Comparing with ADM derived from human skin, animal ADM products are more cost-effective and more frequently applied for large skin defects
[18][21]. Some animal ADM, such as those from bovine, porcine, and fish skin, have been approved by the US Food and Drug Administration
[36][37][38][39,40,41]. For instance, Kerecis™ graft, a newly-approved ADM product from fish skin, is very attractive for wound management because of the anti-inflammatory property of its exclusive omega-3 polyunsaturated fatty acids
[39][42]. Further, the Kerecis™ graft avoids the risk of potential viral and prion transmission, which might be seen in mammalian-derived products
[31][34]. According to recent clinical studies, the Kerecis™ graft can heal acute or chronic deep skin wounds with a shorter healing time than conventional wound treatment
[38][39][41,42].
2.2. Small Intestinal Submucosa (SIS)
SIS is a membranous ECM scaffold derived from porcine small intestine. It has attracted considerable attention in clinical applications for tissue regeneration, mainly because of the good capabilities of activating immune mediators, inducing angiogenesis, and promoting reepithelialization
[4][40][41][7,43,44]. These effects are likely due to the release of growth factors, such as basic fibroblastic growth factor, transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF)
[42][43][45,46]. Mechanistically, SIS can orchestrate wound remodeling by eliciting a response of macrophages towards a M2 phenotype rather than a M1 phenotype, where the M1 phenotype can lead to prolonged inflammation and scarring
[44][45][47,48]. Furthermore, SIS has a unique effect on the inhibition of matrix metalloproteinases
[46][49]. Based on these merits, SIS is applicable for treating chronic wounds, where the microenvironment is harsh and the matrix metalloproteinases are abundant
[43][47][48][46,50,51].
Taking Oasis
® Wound Matrix as an example, the clinical safety and efficiency of this SIS product have been confirmed by a clinical trial, in which 130 chronic leg ulcer cases were involved
[49][52]. In this study, Oasis
® Wound Matrix demonstrated 40% of complete healing at 12 week after treatment, while the standard care group just resulted in 29% of complete healing
[49][52]. In another study, chronic venous ulcers treated with SIS Wound Matrix resulted in a significant decrease in the expression of matrix metalloproteinases and pro-inflammatory cytokines, while the level of TGF-β was significantly increased
[50][53]. These results revealed that SIS Wound Matrix healed chronic wounds by leading the healing process to a more acute wound state
[50][53].
2.3. Acellular Amniotic Membrane (AAM)
Amniotic membrane is the innermost layer of fetal placenta. ECM scaffolds derived from human amniotic membranes, termed AMM, have been commercialized for skin wound healing, such as the SURFFIXX
®, AmnioBand
®, Biovance
®, and EpiFix
® [18][51][21,54]. Featuring anti-bacterial, immunomodulatory, and pain-reducing properties, AAM can significantly promote the healing of various kinds of cutaneous wounds, such as superficial or partial thickness burns, pressure sores, and chronic leg ulcers
[52][53][55,56]. According to a systematic review and meta-analysis study, the safety and efficiency of AAM have been confirmed in the treatment of split-thickness skin graft donor sites
[54][57]. For chronic diabetic foot ulcers, a shorter time to complete healing and a higher proportion of complete healing were observed in the AAM group when compared with the standard wound care group
[55][58].
2.4. Other ECM Membranes
Beyond ADM, SIS and AAM, other ECM membranes have been investigated for skin wound healing, such as decellularized membranes derived from mesothelium and forestomach. Decellularized mesothelium membranes are scaffolds obtained from a simple squamous epithelium, which lines the walls of body cavities, such as the pleura, peritoneum, and pericardium
[14][27][17,30]. In 2020, Alizadeh et al. have developed an ovine decellularized pericardium, which seems appealing for skin regeneration
[14][17]. In another example, Endoform
®, a forestomach-derived ECM membrane, was efficient in inhibiting a broad spectrum of matrix metalloproteinases and has been used for the healing of acute and chronic wounds
[43][56][46,59].