Asymmetric cell division (ACD) of neural stem cells and progenitors not only renews the stem cell population but also ensures the normal development of the nervous system, producing various types of neurons with different shapes and functions in the brain. One major mechanism to achieve ACD is the asymmetric localization and uneven segregation of intracellular proteins and organelles into sibling cells. Recent studies have demonstrated that liquid-liquid phase separation (LLPS) provides a potential mechanism for the formation of membrane-less biomolecular condensates that are asymmetrically distributed on limited membrane regions. Moreover, mechanical forces have emerged as pivotal regulators of asymmetric neural stem cell division by generating sibling cell size asymmetry. In this review, we will summarize recent discoveries of ACD mechanisms driven by LLPS and mechanical forces.
- asymmetric cell division
- phase separation
- mechanical force
- myosin flow
- neural stem cell
- polarity cue
Asymmetric cell division (ACD), which generates daughter cells with distinct fates, is a fundamental mechanism for cell diversity and the normal development of multicellular organisms. The Drosophila neural stem cell neuroblast (NB) is regarded as an important model system for understanding the ACD of stem cells and progenitors. Through ACD, Drosophila NBs each produce a larger daughter cell that inherits the characteristics of NBs and can continue asymmetric division and a smaller ganglion mother cell (GMC) which undergoes terminal differentiation to generate two neurons or glial cells (Figure 1) [1]. Importantly, the molecular mechanisms and signaling pathways that regulate the self-renewal and differentiation of NBs are evolutionarily conserved from Drosophila to mammals [2].
At the metaphase of dividing Drosophila NBs, the PAR complex and its related proteins are distributed on the apical cortex of the cell, whereas cell fate determinants and their adaptor proteins are localized at the basal cortex [2-4]. Instead of being uniformly distributed on the apical or basal half of the cell cortex, these proteins form crescent structures at the opposite poles of the cell, respectively (Figure 1) [5-9]. Meanwhile, actomyosin-mediated contraction of actin filaments beneath the cell cortex and dynein-induced cortical pulling on astral microtubules (MTs) load mechanical forces to the cell, thereby changing its shape and behavior, spindle orientation and positioning, and, finally, generating sibling cells with distinct size and function [10,11]. Recently, the liquid–liquid phase separation (LLPS) of biomolecules has been found to play an important role in the organization and regulation of various cellular membrane-less organelles [12-15]. Several studies reported that the apical and basal crescents in dividing NBs are formed by phase separation of certain proteins [16-18].
In this review, we will briefly summarize the basic mechanism of intrinsic ACD using Drosophila NBs as the paradigm, and then we highlight recent findings of biomolecular LLPS and mechanical forces in regulating ACD of Drosophila NBs.
1. Asymmetric CDell Division of Drosophila Neuroblasts
1.1. Asymmetric Protein Localization during ACD
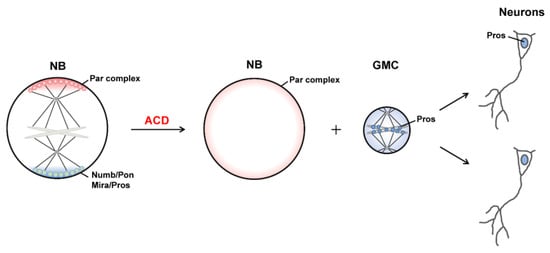
1.2. Generation of Distinct Sibling Cells
2. LLPS and Asymmetric Protein Localization during ACD of NBs
2.1. LLPS in Cells
2.2. LLPS-Mediated Basal Localization of Numb and Pon in Dividing NBs
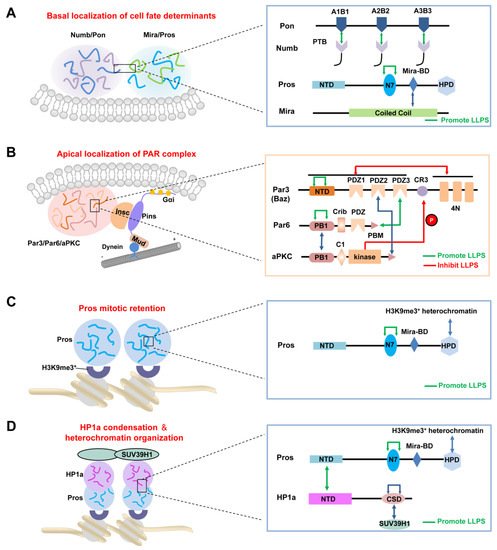
2.3. LLPS-Mediated Apical Localization of the PAR Complexes in Dividing NBs
2.4. LLPS-mediated mitotic implantation of Pros in dividing GMCs
After cytokinesis, the transcription factor Pros enters the GMC nucleus to promote its differentiation [57,58]. Recently, Liu et al. showed that Pros drives irreversible terminal neuronal differentiation by regulating heterochromatin domain condensation and expansion in an LLPS-dependent manner (Figure 2C) [59]. Pros was found to undergo LLPS in vitro and in vivo through self-association through its N7 motif (Figure 2C). LLPS of Pros enabled its retention at histone H3 Lys9 tri-methylation (H3K9me3) heterochromatin regions of chromosomes in mitotic GMCs, where it recruited and concentrated the H3K9me3 “reader” heterochromatin protein 1 (HP1) into the condensed phase via its N-terminal domain (Figure 2D), thus driving the condensation and expansion of the H3K9me3+ heterochromatin regions in the newly generated neurons. After HP1 condensation, Pros, together with a portion of HP1, detached from the H3K9me3+ heterochromatin regions and translocated to its target gene loci, where Pros and HP1 acted cooperatively to silence Pros target genes permanently to drive cell-cycle exit and terminal neuronal differentiation [59]. Pros mutants that exhibited impaired LLPS ability prevented Pros from being retained on chromosomes and thus resulted in compromised terminal differentiation. The above phenotype could be effectively rescued by replacing the N7 motif with another IDR protein capable of LLPS. Interestingly, though the recombinant N7-containing Pros fragment and HP1a co-phase separate in vitro, the Pros condensates and HP1a condensates do not coalesce in vivo [59], suggesting the existence of unknown regulating mechanism(s). Moreover, it is plausible that the basal distribution of Pros in dividing NBs might also be driven by its phase separation, together with the Mira dimer via its coiled-coil domain (Figure 2A).
3. Mechanical forces regulating ACD
Sibling cells generated by ACD of Drosophila NBs have markedly different sizes and components (e.g., polarity proteins and cell fate determinants), thus adopting distinct fates. Such asymmetry can be achieved by cooperative mechanisms in spindle-dependent and independent ways. A spindle-dependent mechanism highly depends on the orientation and positioning of the mitotic spindle, whereas a spindle-independent mechanism involves unequal cortical expansion and correct location of the cleavage furrow of NBs, which is determined by the distribution of non-muscle Myosin II (referred to as Myosin) [11].
3.1. Polarity cue-regulated spindle orientation
During ACD of Drosophila NBs, in addition to the central Pins–Gαi complex that provides a cortical cue for positioning and orientation of the mitotic spindle via the Mud–dynein–dynactin machinery, other regulators have recently been identified. The junctional scaffold Canoe (Afadin in mammals) was found to be a component of the apical Insc–Pins (LGN)–Gαi–Mud (NuMA) super-complex, in which it regulates the spindle orientation by recruiting Mud to the cortex and thus activating the Pins-Mud-dynein pathway in a RanGTP-dependent manner (Figure 3A) [60-62]. Similarly, Afadin regulates the apical-basal spindle orientation during cell division in developing renal tubules [63]. Two independent investigations revealed that the Hippo pathway kinase Warts is involved in this process by phosphorylating both Canoe and Mud to promote Pins-Mud complex-mediated spindle orientation [64,65]. Intriguingly, a recent structural analysis suggested that Afadin binds to LGN in a manner that resembles the Insc–LGN and NuMA–LGN interactions [21,66]. The three components within this complex, Insc, NuMA and Afadin, all interacted with the TPR domain of LGN at the same target binding surface (Figure 3A), which seems contradictory to their physiological function that act cooperatively to mediate spindle orientation.
Recently, the cytosolic tail of the adhesion molecule E-cadherin has been found to act as a cortical cue for spindle orientation by recruiting LGN to cell-cell contacts in MDCK cells [67]. Guided by this spatial information, NuMA was targeted to cell–cell adhesions together with astral microtubules by locally competing for LGN from E-cadherin during mitosis and thus oriented the mitotic spindle [67,68]. As is the case for Afadin, E-cadherin is bound to the same target binding pocket in the TPR of LGN. It remains elusive why so many proteins competitively interact with LGN TPR but exert a cooperative role in mitotic spindle orientation.
3.2 Myosin flows regulated by polarity and spindle cues
Sibling cell size asymmetry mainly results from the biased cortical expansion and controlled cleavage furrow positioning, both of which rely on the dynamic localization of actomyosin and modulation of its contractility [11]. In dividing Drosophila NBs, the intrinsic polarity cue Pins–Gαi has been found to guide the correct localization of myosin spatiotemporally, thus controlling the cleavage furrow position and daughter cell size independent of the mitotic spindle [28,29]. Tsankova et al. recently found that the Rho kinase (Rok) and protein kinase N (Pkn) function sequentially to regulate biased myosin activity and localization in response to Pins in dividing Drosophila NBs (Figure 3B) [69]. Myosin is a substrate of Rok, and Rok-mediated phosphorylation of myosin induces its activation. At the early metaphase, Pins recruits Rok apically, which further concentrates the activated myosin at the apical cortex. Following the apical enrichment of Pkn (via Pins) at the late metaphase, Rok activity is downregulated, and active myosin is dephosphorylated and timely excluded from the apical cortex [69]. As a consequence, the translocation of myosin, which is originated from polarity cues, results in a cortical myosin flow heading the basal cortex and consequent cortical expansion of the apical cortex, which contains fewer myosin filaments with weaker contractile forces [70].
Shortly after the start of the above spindle-independent, basally directed myosin flow (about one minute), another apically directed myosin flow is generated on the basal cortex, which is triggered by the central spindle pathway (Figure 3B) [71]. Microtubules from the central spindle contact the equatorial cortex, leading to localized activation of the small GTPase Rho1 via delivery of the centralspindlin complex at the lateral cortex, which subsequently results in local enrichment and activation of myosin where the cleavage furrow is positioned [71]. Such local enhancement of myosin activity then triggers the basal cortical flow, as the intrinsic contractile property of myosin drives it to move towards the highest myosin density [72]. Consequently, the spindle cue clears myosin from the basal cortex and thus results in the accumulation of myosin at the cleavage furrow. Both the apical and basal cortex expansions are induced by clearance of myosin and relieved actomyosin contractile tension on the apical and basal cortex, respectively. However, the relatively prolonged expansion of the apical cortex results in a larger apical daughter and a smaller basal daughter. A follow-up study suggested that besides the myosin-mediated constriction, intracellular hydrostatic pressure further enhances cortical expansion at the apical cortex at anaphase onset [73].
Taken together, through spatiotemporal polarity and spindle cues, Drosophila NBs establish successive apical and basal cortical myosin flows, relocate myosin to the lateral cortex at anaphase onset, and, thus, determine the cleavage furrow site and enable biased cortical expansion, finally building up physical asymmetry in dividing NBs.
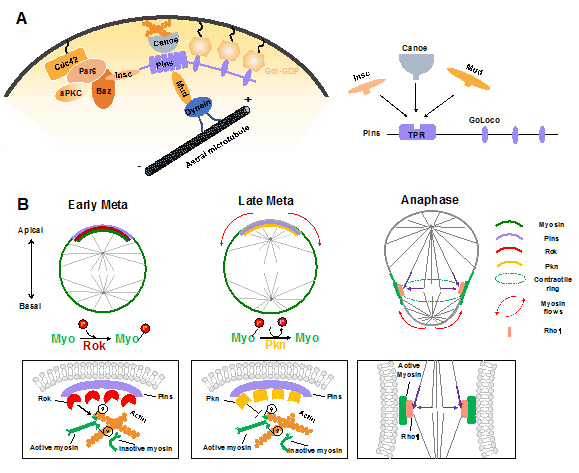
Figure 3. Mechanical forces in regulating ACD of Drosophila NBs. (A) Apical polarity cues Pins and Canoe mediate the assembly of force generators of spindle orientation via guiding the cortical attachment of the Mud–Dynein complex. Intriguingly, Insc, Canoe, and Mud competitively bind to Pins, even though they function cooperatively in the polarity-guided spindle orientation process. (B) Polarity cues and the mitotic spindle regulate spatiotemporal myosin flows to determine biased cortical expansion and cleavage furrow positioning to generate sibling cell size asymmetry. Rok activates myosin through phosphorylation and mediates its cortical localization before mitosis. Pins recruits Rok apically at early metaphase and thereby enriches active myosin at the apical cortex. Subsequently, Pins recruits Pkn apically at late metaphase, leading to timely apical myosin clearance by inhibiting myosin activity. The relief of myosin contraction at the apical cortex leads to its cortical expansion. At anaphase onset, the spindle-mediated accumulation of active myosin at the lateral membrane (via Rho1) promotes the basal myosin clearance and basal cortical expansion. The lateral membrane site with enriched myosin determines the cleavage furrow position.
4.Mechanical Forces Regulating Conclusion and perspectives
Neural stem cells generate sibling cells with distinct sizes, components, and fates through asymmetric division, which can be achieved through interplayed intrinsic mechanisms involving asymmetric localization of polarity cues, regulated spindle orientation, generation of myosin-mediated actin flows, and cleavage furrow positioning. Recent studies have suggested that LLPS is a driving force of the autonomous assembly of the highly enriched protein crescents beneath the apical and basal cortex in dividing Drosophila NBs. Through domain recognition or oligomerization-mediated multivalent interactions, the Baz/Par3–Par-6–aPKC complex and the Numb–Pon complex (likely the Miranda–Pros–Staufen–Brat complex as well) form apical and basal protein condensates spatiotemporally, thus establishing the cell polarity and providing polarity cues for the following ACD-related processes [74]. Compared with the classical membrane anchoring or clustering mechanism, the LLPS theory presents prominent advantages, the most important one being the high dynamics property of protein condensates, which is assumed to be essential for the fast response to cell-cycle signals to assemble or disassemble the cell polarity cues. Moreover, as RNAs with large disordered regions also have high potency for LLPS [75,76], it is plausible that pros mRNA and mira mRNA may further enhance the LLPS property of the Mira-related basal proteins, facilitating their asymmetric localization and segregation during ACD of NBs [25,77].
To generate two distinct sibling cells, the ACD process is accompanied by various forms of mechanical forces arising from precise rearrangements of the actin/microtubule cytoskeleton. Some mechanical forces are highly correlated with polarity cues at the local membrane regions. In this review, we summarize the assembly of the force generator (Pins/Canoe/Mud) for spindle orientation and spatiotemporal generation of myosin flows for biased cortical expansion and cleavage furrow positioning, both of which are regulated by the polarity cue Pins. As Pins is a component of the apical PAR-related super-complex, whether LLPS of polarity cues or other biomolecules plays a regulatory role in the generation and function of intrinsic mechanical forces is an intriguing question. More broadly, whether LLPS and mechanical forces interplay with each other is an interesting research direction.
Several studies have suggested the involvement of biomolecular LLPS in driving the organization of the cytoskeleton. In the presence of Filamin, short actin filaments were recently found to undergo phase separation, whereas long filaments were prone to form gel-like structures via phase transition [78]. Spindle matrix component, BuGZ, phase separated via its C-terminal IDR to promote MT polymerization and assembly of spindle matrix [79]. The MT-binding protein Tau underwent LLPS via its IDR under physiological conditions, whereas neurodegenerative disease-associated hyper-phosphorylation promoted Tau phase transition into gel-like aggregates incapable of MT binding, finally causing neuronal cell death [80-82]. On the other hand, the formation of the condensed crescents during ACD is intimately connected with the mechanical force caused by cortical flows. In dividing Drosophila NBs, actin cytoskeleton-dependent cortical flows facilitated the assembly of the apical aPKC cap at metaphase and its disassembly at anaphase onset [56]. During embryonic polarization in C. elegans, actomyosin contractility and the resulting cortical forces stimulate PAR proteins clustering on the cortex [54].
OACD
3.1. Polarity Cue-Regulated Spindle Orientation
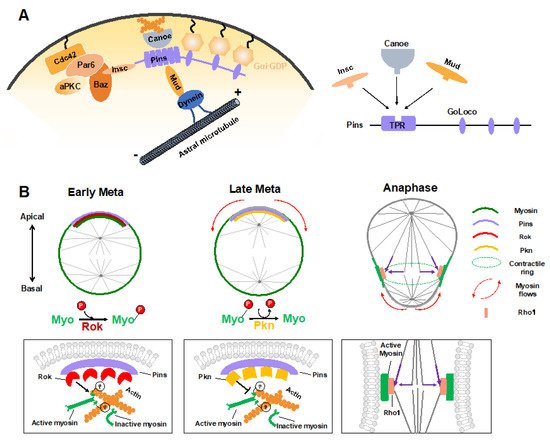
3.2. Myosin Flows Regulated by Polarity and Spindle Cues
References
- Delgado, M.K.; Cabernard, C. Mechanical regulation of cell size, fate, and behavior during asymmetric cell division. Curr. Opin. Cell Biol. 2020, 67, 9–16.
- Speicher, S.; Fischer, A.; Knoblich, J.; Carmena, A. The PDZ Protein Canoe Regulates the Asymmetric Division of Drosophila Neuroblasts and Muscle Progenitors. Curr. Biol. 2008, 18, 831–837.
- Wee, B.; Johnston, C.A.; Prehoda, K.E.; Doe, C.Q. Canoe binds RanGTP to promote PinsTPR/Mud-mediated spindle orientation. J. Cell Biol. 2011, 195, 369–376.
- Choi, W.; Acharya, B.; Peyret, G.; Fardin, M.-A.; Mège, R.-M.; Ladoux, B.; Yap, A.; Fanning, A.S.; Peifer, M. Remodeling the zonula adherens in response to tension and the role of afadin in this response. J. Cell Biol. 2016, 213, 243–260.
- Gao, L.; Yang, Z.; Hiremath, C.; Zimmerman, S.E.; Long, B.; Brakeman, P.R.; Mostov, K.E.; Bryant, D.M.; Luby-Phelps, K.; Marciano, D.K. Developing renal tubules orient cell division via Afadin to position the tubule lumen. Development 2017, 144, 3511–3520.
- Keder, A.; Rives-Quinto, N.; Aerne, B.L.; Franco, M.; Tapon, N.; Carmena, A. The Hippo Pathway Core Cassette Regulates Asymmetric Cell Division. Curr. Biol. 2015, 25, 2739–2750.
- Dewey, E.; Sanchez, D.; Johnston, C.A. Warts Phosphorylates Mud to Promote Pins-Mediated Mitotic Spindle Orientation in Drosophila, Independent of Yorkie. Curr. Biol. 2015, 25, 2751–2762.
- Zhu, J.; Wen, W.; Zheng, Z.; Shang, Y.; Wei, Z.; Xiao, Z.; Pan, Z.; Du, Q.; Wang, W.; Zhang, M. LGN/mInsc and LGN/NuMA Complex Structures Suggest Distinct Functions in Asymmetric Cell Division for the Par3/mInsc/LGN and Gαi/LGN/NuMA Pathways. Mol. Cell 2011, 43, 418–431.
- Carminati, M.; Gallini, S.; Pirovano, L.; Alfieri, A.; Bisi, S.; Mapelli, M. Concomitant binding of Afadin to LGN and F-actin directs planar spindle orientation. Nat. Struct. Mol. Biol. 2016, 23, 155–163.
- Gloerich, M.; Bianchini, J.M.; Siemers, K.A.; Cohen, D.J.; Nelson, W.J. Cell division orientation is coupled to cell–cell adhesion by the E-cadherin/LGN complex. Nat. Commun. 2017, 8, 13996.
- Finegan, T.M.; Bergstralh, D.T. Division orientation: Disentangling shape and mechanical forces. Cell Cycle 2019, 18, 1187–1198.
- Connell, M.; Cabernard, C.; Ricketson, D.; Doe, C.Q.; Prehoda, K.E. Asymmetric cortical extension shifts cleavage furrow position in Drosophila neuroblasts. Mol. Biol. Cell 2011, 22, 4220–4226.
- Cabernard, C.; Prehoda, K.E.; Doe, C.Q. A spindle-independent cleavage furrow positioning pathway. Nat. Cell Biol. 2010, 467, 91–94.
- Tsankova, A.; Pham, T.; Garcia, D.S.; Otte, F.; Cabernard, C. Cell Polarity Regulates Biased Myosin Activity and Dynamics during Asymmetric Cell Division via Drosophila Rho Kinase and Protein Kinase N. Dev. Cell 2017, 42, 143–155.e5.
- Ou, G.; Stuurman, N.; D’Ambrosio, M.; Vale, R.D. Polarized Myosin Produces Unequal-Size Daughters during Asymmetric Cell Division. Science 2010, 330, 677–680.
- Roubinet, C.; Tsankova, A.; Pham, T.; Monnard, A.; Caussinus, E.; Affolter, M.; Cabernard, C. Spatio-temporally separated cortical flows and spindle geometry establish physical asymmetry in fly neural stem cells. Nat. Commun. 2017, 8, 1–16.
- Mayer, M.; Depken, M.; Bois, J.; Julicher, F.; Grill, S.W. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nat. Cell Biol. 2010, 467, 617–621.
- Pham, T.T.; Monnard, A.; Helenius, J.; Lund, E.; Lee, N.; Müller, D.J.; Cabernard, C. Spatiotemporally Controlled Myosin Relocalization and Internal Pressure Generate Sibling Cell Size Asymmetry. iScience 2019, 13, 9–19.
- Boeynaems, S.; Alberti, S.; Fawzi, N.L.; Mittag, T.; Polymenidou, M.; Rousseau, F.; Schymkowitz, J.; Shorter, J.; Wolozin, B.; van den Bosch, L.; et al. Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 2018, 28, 420–435.
- Alberti, S.; Hyman, A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 196–213.
- Roden, C.; Gladfelter, A.S. RNA contributions to the form and function of biomolecular condensates. Nat. Rev. Mol. Cell Biol. 2021, 22, 183–195.
- Hyman, A.A.; Weber, C.A.; Julicher, F. Liquid-Liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58.
- Zhao, Y.G.; Zhang, H. Phase Separation in Membrane Biology: The Interplay between Membrane-Bound Organelles and Membraneless Condensates. Dev. Cell 2020, 55, 30–44.
- Quiroz, F.G.; Fiore, V.F.; Levorse, J.; Polak, L.; Wong, E.; Pasolli, H.A.; Fuchs, E. Liquid-liquid phase separation drives skin barrier formation. Science 2020, 367, eaax9554.
- Ong, J.Y.; Torres, J.Z. Phase Separation in Cell Division. Mol. Cell 2020, 80, 9–20.
- Wu, X.; Cai, Q.; Feng, Z.; Zhang, M. Liquid-Liquid Phase Separation in Neuronal Development and Synaptic Signaling. Dev. Cell 2020, 55, 18–29.
- Tsang, B.; Pritišanac, I.; Scherer, S.W.; Moses, A.M.; Forman-Kay, J.D. Phase Separation as a Missing Mechanism for Interpretation of Disease Mutations. Cell 2020, 183, 1742–1756.
- Zhang, H.; Ji, X.; Li, P.; Liu, C.; Lou, J.; Wang, Z.; Wen, W.; Xiao, Y.; Zhang, M.; Zhu, X. Liquid-liquid phase separation in biology: Mechanisms, physiological functions and human diseases. Sci. China Life Sci. 2020, 63, 953–985.
- Jain, A.; Vale, A.J.R.D. RNA phase transitions in repeat expansion disorders. Nat. Cell Biol. 2017, 546, 243–247.
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.; et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162, 1066–1077.
- Wang, Z.; Zhang, H. Phase Separation, Transition, and Autophagic Degradation of Proteins in Development and Pathogenesis. Trends Cell Biol. 2019, 29, 417–427.
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29.
- Posey, A.E.; Holehouse, A.S.; Pappu, R.V. Phase Separation of Intrinsically Disordered Proteins. Methods Enzymol. 2018, 611, 1–30.
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298.
- Banani, S.F.; Rice, A.; Peeples, W.B.; Lin, Y.; Jain, S.; Parker, R.; Rosen, M.K. Compositional Control of Phase-Separated Cellular Bodies. Cell 2016, 166, 651–663.
- Zeng, M.; Shang, Y.; Araki, Y.; Guo, T.; Huganir, R.L.; Zhang, M. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell 2016, 166, 1163–1175.e12.
- Li, P.; Banjade, S.; Cheng, H.-C.; Kim, S.; Chen, B.; Guo, L.; Llaguno, M.; Hollingsworth, J.V.; King, D.S.; Banani, S.F.; et al. Phase transitions in the assembly of multivalent signalling proteins. Nat. Cell Biol. 2012, 483, 336–340.
- Nott, T.; Petsalaki, E.; Farber, P.; Jervis, D.; Fussner, E.; Plochowietz, A.; Craggs, T.; Bazett-Jones, D.P.; Pawson, T.; Forman-Kay, J.D.; et al. Phase Transition of a Disordered Nuage Protein Generates Environmentally Responsive Membraneless Organelles. Mol. Cell 2015, 57, 936–947.
- Shan, Z.; Tu, Y.; Yang, Y.; Liu, Z.; Zeng, M.; Xu, H.; Long, J.; Zhang, M.; Cai, Y.; Wen, W. Basal condensation of Numb and Pon complex via phase transition during Drosophila neuroblast asymmetric division. Nat. Commun. 2018, 9, 1–16.
- Liu, Z.; Yang, Y.; Gu, A.; Xu, J.; Mao, Y.; Lu, H.; Hu, W.; Lei, Q.-Y.; Li, Z.; Zhang, M.; et al. Par complex cluster formation mediated by phase separation. Nat. Commun. 2020, 11, 1–18.
- Lu, B.; Ackerman, L.; Jan, L.; Jan, Y.-N. Modes of Protein Movement that Lead to the Asymmetric Localization of Partner of Numb during Drosophila Neuroblast Division. Mol. Cell 1999, 4, 883–891.
- Patel, S.S.; Belmont, B.; Sante, J.M.; Rexach, M.F. Natively Unfolded Nucleoporins Gate Protein Diffusion across the Nuclear Pore Complex. Cell 2007, 129, 83–96.
- Kono, K.; Yoshiura, S.; Fujita, I.; Okada, Y.; Shitamukai, A.; Shibata, T.; Matsuzaki, F. Reconstruction of Par-dependent polarity in apolar cells reveals a dynamic process of cortical polarization. eLife 2019, 8, 8.
- Wilson, M.I.; Gill, D.J.; Perisic, O.; Quinn, M.; Williams, R.L. PB1 Domain-Mediated Heterodimerization in NADPH Oxidase and Signaling Complexes of Atypical Protein Kinase C with Par6 and p62. Mol. Cell 2003, 12, 39–50.
- Soriano, E.V.; Ivanova, M.E.; Fletcher, G.; Riou, P.; Knowles, P.P.; Barnouin, K.; Purkiss, A.; Kostelecky, B.; Saiu, P.; Linch, M.; et al. aPKC Inhibition by Par3 CR3 Flanking Regions Controls Substrate Access and Underpins Apical-Junctional Polarization. Dev. Cell 2016, 38, 384–398.
- Holly, R.W.; Jones, K.; Prehoda, K.E. A Conserved PDZ-Binding Motif in aPKC Interacts with Par-3 and Mediates Cortical Polarity. Curr. Biol. 2020, 30, 893–898.e5.
- De Sá, E.M.; Mirouse, V.; Johnston, D.S. aPKC Phosphorylation of Bazooka Defines the Apical/Lateral Border in Drosophila Epithelial Cells. Cell 2010, 141, 509–523.
- Wang, S.-C.; Low, T.Y.F.; Nishimura, Y.; Gole, L.; Yukako, N.; Motegi, F. Cortical forces and CDC-42 control clustering of PAR proteins for Caenorhabditis elegans embryonic polarization. Nat. Cell Biol. 2017, 19, 988–995.
- Rodriguez, J.; Peglion, F.; Martin, J.; Hubatsch, L.; Reich, J.; Hirani, N.; Gubieda, A.G.; Roffey, J.; Fernandes, A.R.; Johnston, D.S.; et al. aPKC Cycles between Functionally Distinct PAR Protein Assemblies to Drive Cell Polarity. Dev. Cell 2017, 42, 400–415.e9.
- Oon, C.H.; Prehoda, K.E. Asymmetric recruitment and actin-dependent cortical flows drive the neuroblast polarity cycle. eLife 2019, 8, 8.
