Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Marc Port and Version 2 by Bruce Ren.
In the diagnostic field, preclinical proofs of concept have been demonstrated for X-ray, photoacoustic and fluorescence imaging. In the therapeutic field, several preclinical studies have shown the potential of bismuth nanoparticles as X-ray radiosensitizers for use in radiotherapy and as photothermal agents for applications in near infrared phototherapy.
- bismuth
- nanoparticles
- theranostic agents
- biocompatibility
1. Introduction
Recently, the synthesis of metallic bismuth nanoparticles (Bi NPs) has been reviewed by our group. Despite the interest in nanoparticle technologies, only approximately fifty papers have described metallic Bi NP production [1], while many works describe the synthesis and biomedical applications of non-metallic bismuth nanoparticles such as bismuth oxyhalides and bismuth chalcogenides, including bismuth oxide, bismuth sulfide, bismuth selenide, and bismuth telluride [2]
Bismuth is a diamagnetic semimetal with a very small band gap. This material shows several interesting properties, such as high magnetoresistance, thermal conductivity and high anisotropic electronic behaviour [3]; these properties prompted researchers to synthesize Bi NPs for electronic applications. Bi NPs have also been studied as chemical catalysts. Recently synthesized Bi NPs have proven to be efficient, when used with NaBH4, for reducing 4-nitrophenol [4][5][4,5]. On the other hand, Cui et al. characterized the photocatalytic activity of Bi NPs [6][7][6,7].
Bismuth(III) complexes are used in medicine. For example, bismuth subsalicylate is used for the relief of diarrhoea and upset stomach due to overindulgence in food and drink. This single-dose medicine contains milligram quantities of bismuth(III) in complex with salicylate. Another bismuth(III) complex, bismuth subcitrate potassium, is used in combination with antibiotics and proton pump inhibitors for the treatment of Helicobacter pylori infections.
Despite this clinical use of bismuth complexes, only a few recently published studies, between 2012 and 2018, have described the medical theranostic applications of metallic Bi NPs, and the purpose of this paper is to provide an exhaustive review of these studies (Figure 1):
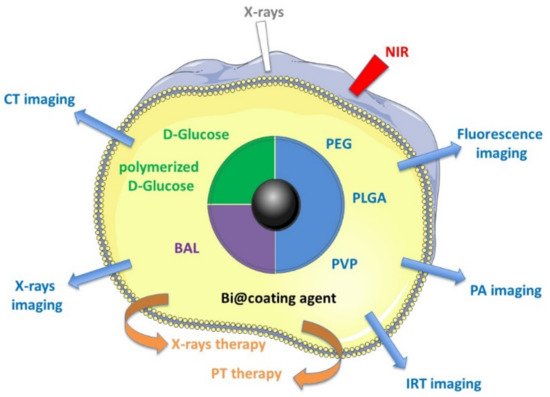
Figure 1. Medical theranostic applications described with different Bi NPs.
2. Metallic Bismuth Nanoparticles as Imaging Contrast Agents
The metallic and nanometric properties of Bi NPs have enabled the generation of proofs of concept for their use as contrast agents in different imaging modalities: X-ray, fluorescence and photoacoustic visualization (Table 1).
Table 1. Metallic bismuth nanoparticles as imaging contrast agents and as X-ray radiosensitizers, for theranostic applications.
| Entry | Capping Agent | Diameter TEM (nm) | Biological Applications | Proof of Concept | Reference |
|---|---|---|---|---|---|
| 1 | PVP, APTES and conjugation with folic acid | 30 | X-ray radiosensitizers to detect and kill circulating tumor cells | In vitro | Hossain et al. 2012 |
| 2 | PVP and conjugation with Pseudomonas aeruginosa polyclonal antibody | 30 | X-ray radiosensitizers to eliminate bacteria | In vitro | Luo et al. 2013 |
| 3 | Red blood cell membrane and conjugation with folic acid | 56 | X-ray radiosensitizers for breast cancer | In vitro & in vivo (mice) | Deng et al. 2018 |
| 4 | Cellulose nanofiber | 2–10 | X-ray radiosensitizers for breast cancer | In vitro & in vivo (mice) | Jiao et al. 2018 |
| 5 | 1-Dodecanethiol PEGylated phospholipid | 40 | CT tomography & photothermal and radiotherapy treatment of tumors. | In vitro & in vivo (mice) | Yu et al. 2018 |
| 6 | DSPE-PEG5000 and conjugation to peptide LyP-1 | 3.6 | CT tomography & photoacoustic imaging agent & NIR-photothermal and radiotherapy treatment of tumors. | In vitro & in vivo (mice) | Yu et al. 2017 |
| 7 | DLPC (,2-dilauroyl-sn-glycero-3-phosphocholine) | 47 | CT tomography & photoacoustic imaging agent & NIR-photothermal treatment of tumors. | In vitro & in vivo (mice) | Yang et al. 2018 |
| 8 | Poly (vinylpyrrolidone) | 2.7 | CT &photothermal-imaging-guided photothermal therapy | In vitro & in vivo (mice) | Lei et al. 2017 |
| 9 | Ppy PEG | 70 | CT tomography & photoacoustic imaging agent & NIR-photothermal treatment of tumors | In vitro & in vivo (mice) | Yang Sisi et al. 2017 |
| 10 | DSPE PEG | 100 **** | CT tomography & photoacoustic imaging agent & NIR-photothermal treatment of tumors | In vitro & in vivo (mice) | Lu et al. 2019 |
| 11 | GEL, BSA, HSA | 15–19 | CT tomography & infrared thermal & antitumor PTT | In vitro & in vivo (mice) | Liu et al. 2020 |
| 12 | PEG | 41 | Trimodal imaging (CT, photoacoustic and infrared thermal) & antitumor PTT | In vitro & in vivo (mice) | Li et al. 2017 |
| 13 | d-glucose & 1,2-propanediol | 74 | CT tomography | In vitro | Brown et al. 2014 |
| 14 | PLGA and SiO2 | 12 | CT tomography | In vitro | Chakravarty et al. 2016 |
| 15 | PLGA | 120 * | CT tomography | In vitro & ex vivo (chicken wing forearm) | Swy et al. 2014 |
| 16 | PEG NH2 | 4 to 100 | CT tomography and fluorescence imaging | In vitro & in vivo (mice) | Bi et al. 2018 |
| 17 | Polymerized d-glucose | 22 | CT tomography (GI tract) | In vitro & in vivo (mice) | Wei et al. 2016 |
| 18 | BSA | 6–7 | CT tomography, fluorescence imaging and cytotoxicity | In vitro & in vivo (mice) | Liu et al. 2017 |
| 19 | Surfactant (not described) | 10–100 * | CT tomography and radiotherapy | In vivo (mice) | Torisi et al. 2018 |
| 20 | Mesoporous silica | 115 nm **** | CT tomography/magnetic resonance imaging chemo/photothermal/chemodynamic therapy |
In vitro & in vivo (mice) | Zhao et al. 2021 |
In general, nanoparticles used for medical imaging are characterized by an increased blood residence time, as their leakage across capillary vessels is limited. Thus, these nanoparticles are well suited for imaging vessels and their abnormalities [8][9][10][11][8,9,10,11].
2.1. Metallic Bismuth Nanoparticles as Contrast Agents for X-ray Imaging
Bismuth has the highest atomic number among “nonradioactive elements”, and is characterized by the highest X-ray absorption among the heavy metals at any energy of incident X-ray photons. Consequently, bismuth compounds are attractive for designing new X-ray contrast agents (XCAs). It is particularly interesting that, because of its high atomic number (Z = 83), bismuth has enhanced X-ray opacity compared to that of the clinically approved iodine-based (Z = 53) or barium (Z = 56) XCAs [8].
Bismuth oxide (Bi2O3) and bismuth sulphide (Bi2S3) nanoparticles have been extensively studied in imaging. However, to be an efficient XCA, a high density of metal atoms must be contained inside the nanoparticle. Therefore, the drawback of Bi2O3 and Bi2S3 is the lower concentration of bismuth atoms per particle because of the oxygen or sulphur content. Moreover, the low stability and aggregation tendency of these particles is evident in physiological environments. The instability of Bi2S3 in aqueous media is problematic because the observed hydrolysis leads to toxic hydrogen sulphide gas. Consequently, metallic Bi NPs are particularly attractive as XCAs because they contain only bismuth atoms, which attenuate X-rays in a relatively small volume and are thus characterized by a high density of atoms opaque to X-rays. Bi NPs are sometimes compared to the well-studied gold nanoparticles (Au NPs) because both are easily synthesized with different sizes and morphologies [12]. However, gold is currently approximately 2000-fold more expensive than bismuth.
The potential of different kinds of radiopaque metallic Bi NPs as high-contrast, long-circulating XCAs was recently explored in four studies.
2.1.1. d-Glucose or Polymerized d-Glucose Coatings
In 2014, the synthesis of Bi NPs coated with d-glucose (Bi@d-glucose) was described [13]. These Bi NPs contain around 6 million bismuth atoms per nanoparticle and are characterized by a very dense bismuth core that constitutes the majority (~60%) of the particle volume. Quantitative computed tomography (CT) using phantoms has demonstrated that Bi@d-glucose NPs have greater X-ray opacity than clinical, iodinated contrast agents (iopamidol, a marketed iodinated XCA) at the same concentration. Moreover, Bi NP attenuation is relatively insensitive to the range of tube voltages used in clinical CT scanners (80 to 140 kV), which is advantageous because the same contrast is produced using any CT imaging protocol. The imaging of cells (HeLa cells and murine macrophages) incubated with different Bi NP concentrations enabled an in vitro quantitative analysis of CT attenuation. CT imaging also revealed that the uptake by both types of cells had a linear correlation with XCA concentration, indicating a nonspecific uptake process. As expected, this uptake was more pronounced with the murine macrophage line, which is consistent with its greater phagocytic activity.
The preparation of highly monodisperse aqueous Bi NPs coated with polymerized d-glucose was reported in 2016 [14]. The CT contrast efficiency of these Bi NPs was evaluated in comparison with that of a BaSO4 suspension in vitro. The Bi NPs produced much higher CT contrast per unit of mass concentration than did BaSO4, regardless of the CT scanner operating voltage (80 kV and 120 kV). The high stability of these Bi NPs allowed for their oral or rectal administration to mice to achieve CT imaging of the gastrointestinal (GI) tract. After oral administration of these Bi NPs, the upper GI tract and the arrangement of the small intestinal loops were visualized with high contrast. Rectal administration enabled the visualization of the lower GI tract (the rectum and descending colon).
2.1.2. PLGA Coating
Chakravarty et al. studied two kinds of Bi NPs obtained by complex procedures: Bi Ganex (BiG) nanocrystals encapsulated by poly (DL-lactic-co-glycolic acid) (BiG@PLGA) and by a SiO2 coating (BiG@SiO2) [15]. These two kinds of Bi NPs were dispersed in a 0.5% agarose gel. These phantoms were imaged by a CT scanner operating at a tube voltage of 80 kV and compared with those generated with iopamidol (a marketed iodinated XCA) at various concentrations (0 to 80 mM). The CT contrast enhancement of both Bi NPs was threefold that of Iopamidol (300 mg/mL) at isoconcentration, as demonstrated by the Hounsfield units (HU) quantified with respect to the bismuth or iodine concentration.
Swy et al. used poly (DL-lactic-co-glycolic acid) (PLGA) to encapsulate the Bi NPs [16]. The resulting encapsulated NP had a diameter of 120 nm. After 24 h in an acidic solution imitating the lysosomal medium, the Bi@PLGA NPs showed nearly 70% degradation, whereas in cytosol- and extracellular fluid-imitating media, they remained completely stable. Both a clinical imager and a μCT imager detected these Bi NPs in vitro. The rate of attenuation was higher using μCT because the low-energy component of the μCT X-ray beam was greater than that of the clinical CT system. The ability to detect Bi NPs ex vivo by CT and μCT was also demonstrated by injecting Bi NPs into a chicken wing forearm.
2.1.3. BSA Coating
Bovine serum albumin (BSA)-coated Bi NPs (hydrodynamic diameter of 62 nm) were imaged by CT imaging, operating at a tube voltage of 60 kV [17]. Bi NPs were intravenously injected in mice subcutaneously transplanted with mammary carcinoma tumour cell line 4T1. One hour later, in vivo CT imaging of the mice showed an enhanced contrast of tumour due to Bi NP accumulation, because of the high permeability and retention effect of the tumour.
2.2. Metallic Bismuth Nanoparticles for Dual-Modal Imaging: X-ray and Fluorescence
Bi et al. rendered small Bi NPs water soluble by means of a polyethylene glycol (PEG) coating and used TEM to characterize their diameter as 4 nm [18]. The originality of this work highlights the fluorescent properties of the Bi@PEG NPs and, thus, suggests them as new types of NP for dual-modal X-ray and fluorescence imaging. The spectral emission of the Bi@PEG NPs was studied and a maximum effect was observed at an excitation wavelength of 525 nm. The in vitro CT imaging efficiency was evaluated by determining the HU values. In vivo CT imaging was performed after the intravenous injection of the Bi NPs into mice, with the Bi NPs demonstrating a long circulation time; this property is due to the PEG coating inducing greater Bi NP accumulation in the liver and intestine than was realized with 300 mg/mL iohexol (a marketed iodinated XCA). The in vivo fluorescence imaging was conducted by using a 600 nm excitation wavelength. After Bi NP injection, the fluorescence signal was detected in the chest, epigastrium and, gradually, in the hypogastrium, observations consistent with the CT images.
Bismuth nanoparticles obtained by laser ablation and coated with a non-described surfactant were injected with a micro syringe at a concentration of 1 mg/mL in specific organs of healthy mice [19]. Mice were immediately submitted to X-ray tube irradiation (20–45 kV) in order to provide contrasted fluorescence images acquired with a fast CCD camera. The fluorescence image displays the organ and the nearest blood irrigated tissues very well. This imaging procedure allowed for description of the spatial location of the Bi-NPs as a function of the time from the injection instant.
2.3. Metallic Bismuth Nanoparticles for Photoacoustic Imaging
Photoacoustic imaging (PAI) is an interesting non-invasive imaging modality that combines the spectral selectivity of molecular excitation by laser light with the high resolution of ultrasound imaging. The photoacoustic effect is due to the generation of an acoustic wave detected by a transducer and the absorption of optical energy. Compared with fluorescence optical imaging, PAI has a higher spatial resolution (as low as 5 μm) and greater imaging depth (up to 5–6 cm) because the scattering of ultrasonic signals is much weaker than that of light in tissue. Compared with ultrasound imaging, in which contrast is limited by the mechanical properties of the biological tissues, PAI has better tissue contrast, which is related to the optical properties of the different tissues. Metallic NPs, especially Au NPs, have been recently used as PAI contrast agents due to the optical absorption caused by their surface plasmon resonance (SPR) effect. The SPR effect occurs when free charges on the nanoparticle surface oscillate with the electromagnetic field, leading to strong optical absorption.
Four different teams demonstrated that the SPR effect of Bi NPs can generate a signal detectable with PAI [20][21][22][23][20,21,22,23]. These papers address multimodal therapy and consequently will be analysed in detail in Section 4.2.
All these examples demonstrate that Bi NPs are efficient objects for obtaining multimodal contrast agents. Their intrinsic CT, PA and fluorescence imaging modalities can be combined. However, the proof of concept remains preclinical. The biodistribution and diffusion of these objects is probably limited by their nanoparticulate nature, and will require adjustments to consider their clinical use.
3. Metallic Bismuth Nanoparticles as X-ray Radiosensitizers
Radiotherapy (RT), an effective medical strategy complementary to chemotherapy and surgery that enables the treatment of solid tumours and distant or locoregional metastases, is currently used to treat more than half of cancer patients [24]. The radiation used in RT can indirectly or directly damage tumour cell targets by producing free radicals which induce the increased production of toxic reactive oxygen species (ROS). The difficulties in applying this technique are based on the similar mass energy absorption characteristics of the healthy and cancerous tissues. Improving tumour cell sensitivity to RT remains a major clinical challenge to treating radiation-resistant tumours and to limiting the doses received by healthy organs located near tumours. To sensitize tumours to radiation, NPs consisting of high-Z elements have been demonstrated to function as powerful radiosensitizers in RT during preclinical and clinical trials [24]. Indeed, elements such as gold, platinum, silver, gadolinium, iron and hafnium incorporated into NPs have a large cross-section for radiation absorption and photoelectron or Auger electron generation. These NPs significantly increase the deposited dose in their vicinity because of their high-energy absorption coefficients [12]. Consequently, these metallic NPs are able to concentrate higher radiation doses within tumours, thus enhancing RT efficacy and reducing the risk of possible side effects.
In an initial work, Hossain et al. made a mathematical model to compare the performance of different metallic NPs (bismuth, gold and platinum) as radiosensitizers [25]. Mathematical models quantified the dose enhancement factor, which represents the ratio of the delivered dose to cells with and without NPs. According to this model, Bi NPs provide higher dose enhancements than Au or Pt NPs for a given nanoparticle size, concentration and location. No experimental in vitro or in vivo work was undertaken to support the predictive data in this study.
However, in another paper published in 2012, Hossain et al. described an innovative technique based on the simultaneous use of two kinds of NPs, superparamagnetic Fe oxide NPs and X-ray absorbing Bi NPs. They detected and killed circulating tumour cells released inside the blood stream of patients during cancer development, confronting a major problem in cancer metastasis management [26]. This technique enables the use of an integrated enrichment system, early detection and circulating tumour cell eradication. An in vitro proof of concept demonstrated the feasibility of this approach by simultaneously using the two kinds of NPs, both of which were modified by folic acid ligands that bind to folate receptors overexpressed on tumour cell surfaces. After adding both NPs to the cell suspension, the NPs bound to the surfaces of tumour cells. The use of superparamagnetic Fe oxide NPs allows a micromagnet to be used to localize and immobilize the circulating tumour cells in a small area. Bi NPs enable circulating tumour cells to be detected by X-rays and fluorescence, then radiosensitized, and finally, killed by increasing the X-ray intensity.
Very recently, four teams demonstrated the potential of Bi NPs as radiosensitizers to improve cytotoxic ROS production by radiotherapy [21][27][28][29][21,27,28,29]. In this section, the work of Deng et al. [27] and Jiao et al. [28] are discussed, while other studies are analysed in Section 4.2.
Metallic Bi NPs coated by cellulose nanofibres (TEM diameter between 2 and 10 nm) were prepared by Jiao et al. [28]. These Bi NPs showed low cytotoxicity when administered alone, but induced concentration-dependent cytotoxicity upon exposure to 10 Gy X-ray radiation, which was indicated by the production of ROS at high yield. To assess the potential of Bi NPs in X-ray radiotherapy in vivo, 4T1 tumour-bearing BALB/C mice were injected intratumorally (100 μL of 2 mg/mL Bi NPs) and then exposed to X-ray radiation (10 Gy). The tumours grew rapidly in the control groups (PBS, PBS with irradiation, Bi NPs without irradiation), but in the presence of Bi NPs under X-ray irradiation, tumour growth was significantly inhibited.
Deng et al. prepared folate-inserted, red blood cell membrane (RBC)-modified Bi NPs (Bi@F-RBC NPs) coated with glucose (TEM diameter: 56 nm) [27]. This particular nanoconstruction allowed for the fine-tuning of the pharmacokinetics, biodistribution and efficacy of the radiosensitizers. Indeed, the incorporation of Bi NPs in RBCs showed a long blood circulation time, whereas folic acid incorporation enabled the targeting of the folate receptor, which is overexpressed in breast cancer. The in vitro X-ray radiotherapy efficiency was demonstrated by the measurement of ROS production (carboxy-H2DCFDA assay) in the 4T1 tumour cells incubated with Bi@F-RBC NPs. A sixfold increase in ROS production was observed in the cells treated with Bi@F-RBC NPs and exposed to X-ray radiation compared to the level observed in the untreated cells. An increase in residual DNA double-strand breaks (H2AX staining) was shown after enhanced X-ray radiation by using Bi@F-RBC NPs. Due to these encouraging results, an in vivo study was conducted on 4T1 tumour cell-bearing BALB/C mice. After intravenous injection of Bi@F-RBC NPs (100 mL of 4 mg/mL Bi NPs) and X-ray irradiation at a dose of 9 Gy, the changes in tumour volume of the mice were monitored. After irradiation, the Bi@F-RBC NPs significantly inhibited tumour growth. The average tumour weight in the mice treated with Bi@F-RBC NPs and exposed to radiation was 6.6-fold lower than that of the mice treated with PBS alone. The in vivo biodistribution and histological analysis indicated that the Bi@F-RBC NPs were excreted from the animal body after 15 days, and no evident damage or inflammation was observed in the major organs.
An original microbiological application of Bi NPs as radiosensitizers was described as significantly damaging to bacterial DNA [30]. Indeed, these Bi NP radiosensitizers were used to induce free radicals and photoelectrons upon X-ray irradiation. The proof of concept of this methodology was demonstrated in vitro on the multidrug-resistant bacterium Pseudomonas aeruginosa by using Bi NPs conjugated to a polyclonal antibody specifically targeting bacterial surfaces. After exposure to 40 kVp X-rays for 10 min, no significant harmful effects on human cells (HeLa and MG-63 cells) were observed. Ninety percent of the bacteria were killed in the presence of these Bi NPs (200 mg mL−1), whereas only 6% were killed in the absence of Bi NPs.
These different studies clearly show the obvious potential of Bi NPs as radiosensitizers. However, it remains necessary to demonstrate the clinical interest of Bi NPs, in particular in comparison to hafnium oxide nanoparticles (NBTXR3, Hensify R), already authorized for their use in humans to treat soft tissue sarcomas.
4. Metallic Bismuth Nanoparticles for Theranostic Applications
4.1. Metallic Bismuth Nanoparticles for Dual X-ray Contrast and NIR-Photothermal Therapy (Thermoradiotherapy)
Near infrared (NIR)-based photothermal therapy (PTT) is currently under preclinical and clinical investigation, especially to determine its suitability as a cancer treatment. PTT employs a photothermal agent to generate heat, causing irreversible tumour tissue damage upon NIR laser irradiation. The wavelength of the NIR is minimally absorbed by blood and soft tissues, leading to deep penetration of the laser. Compared to conventional therapeutic modalities, PTT exhibits unique advantages as a cancer therapy, including high specificity, minimal invasiveness and precise spatial-temporal selectivity. The therapeutic efficacy of PTT significantly depends on the transformation of light by photothermal agents to obtain sufficient heat. Metallic NPs composed of gold, silver, palladium or platinum have been described as efficient photothermal agents as, after NIR excitation, they release vibrational energy and, consequently, heat.
Metallic Bi NPs coated with poly (vinylpyrrolidone) (PVP) were used for dual-modal CT and photothermal-imaging-guided PTT [31]. These Bi@PVP NPs could be dispersed in water and showed strong NIR absorbance, a high photothermal conversion efficiency and photostability. The efficiency of these Bi@PVP NPs in CT was demonstrated in vitro, as the Bi NPs produced a higher contrast than iobitridol (a marketed iodinated XCA) at equivalent concentrations. This CT performance was confirmed by CT imaging of UT4 tumour-bearing Balb/c mice intravenously injected with Bi@PVP NPs. Then, in the same animal model, the in vivo PTT effect was demonstrated as a significantly elevated temperature (measured by infrared photothermal imaging at the tumour site). The intravenous injection of 20 mg kg−1 Bi@PVP NPs was performed after irradiation with an 808 nm laser (1.3 W cm−2). The hyperthermic effect induced by PTT induced complete tumour inhibition at 14 days.
4.2. Metallic Bismuth Nanoparticles for Multimodal Imaging and Multimodal Therapy
4.2.1. X-ray Imaging and PTT/RT
The theranostic potential of Bi NPs was demonstrated by evaluating both their diagnostic efficiency in X-ray imaging and their therapeutic efficacy in PTT and RT [29]. For these purposes, uniform and stable lipophilic Bi NPs (TEM diameter: 40 nm) coated with 1-dodecanethiol ligands and encapsulated by amphiphilic PEGylated phospholipid (DSPE-PEG) were prepared and named Bi@SR-PEG NPs. HU quantification demonstrated that these Bi@SR-PEG NPs provided better CT imaging signals than did iobitridol (a marketed iodinated XCA). Subcutaneous 4T1 tumour-bearing mice were intravenously (IV) injected with Bi@SR-PEG NPs (200 mL, 2.0 g L−1). CT imaging enabled the detection of the tumour area with a maximum contrast obtained 1 h post-injection, before the washed-out phase. A long-lasting liver contrast enhancement was observed, reflecting the phagocytosis of Bi@SR-PEG NPs by Kupffer cells. Then, Bi@SR-PEG was evaluated first in vitro for its performance in NIR-PTT and RT applications. High NIR absorbance and photothermal efficiency were observed under irradiation with an 808-nm laser (1.0 W cm−2) for 10 min. The NIR-PTT potential was confirmed in vitro in the 4T1 cells. After the 4T1 cells were incubated with Bi@SR-PEG NPs (40 mg L−1) and exposed to an 808-nm laser (intensity of 1.0 W cm−2 for 10 min), a negligible number of stained living cells were observed. The in vitro efficiency of Bi@SR-PEG NPs as X-ray radiosensitizers was demonstrated by using a clonogenic survival assay. After incubation of 4T1 cells with Bi@SR-PEG NPs (40 mg L−1) and exposure to X-ray irradiation (4 Gy), the survival fraction of the 4T1 cells was determined to be 0.27 and 0.57 in the presence and absence of Bi-SR-PEG, respectively. The sensitization effect was similar, and these promising in vitro results encouraged the authors to test the synergistic effect of Bi@SR-PEG NPs in NIR PTT and RT in vivo. Subcutaneous 4T1 tumour-bearing mice were intravenously injected with Bi@SR-PEG NPs (200 mL, 2 g L−1) and then exposed, successively, to an 808-nm laser with an intensity of 1.0 W cm−2 for 10 min and to X-ray irradiation (4 Gy). After 14 days, the mice were sacrificed. This synergistic dual treatment induced the inhibition of tumour growth (determined by measuring the average tumour volume) and cell apoptosis (determined by cell morphology and haematoxylin and eosin staining). Individual therapy (NIR-PTT or RT) had a limited therapeutic effect compared with the synergistic effect of the combined NIR-PTT and RT treatment, as demonstrated in this publication.
4.2.2. X-ray CT/PA Imaging and PTT/RT
Yu et al. described the synthesis and biological evaluation of a multifunctional theranostic agent based on a peptide (LyP-1)-labelled Bi NP (Bi@LyP-1 NP) [21]. The ability to absorb both ionizing radiation and laser radiation ensured that Bi@LyP-1 NPs could be used for dual-modal computed tomography and photoacoustic imaging and the synergistic NIR-photothermal and radiotherapy treatment of tumours. By evaluating the HU values, it was demonstrated that the CT signal enhancement capability of Bi@LyP-1 NPs in vitro was superior to that of iohexol (a marketed iodinated XCA). This was further confirmed in vivo by CT imaging after the intratumoural injection of Bi@LyP-1 NPs into 4T1 tumour-bearing mice. As Bi@LyP-1 NPs enable strong and broad NIR absorption, it was possible to study the potential of the Bi NPs with PAI. After the injection of Bi@LyP-1 NPs intratumorally or intravenously into mice bearing subcutaneous 4T1 cell tumours, high photoacoustic signal intensity was observed in the tumours under a wide range of NIR wavelengths (from 700 to 900 nm). The in vitro performance of the Bi@LyP-1 NPs in NIR PTT and RT was then assessed. The Bi@LyP-1 NPs exhibited a broad absorption, between 200 and 2000 nm, and were capable of acting as NIR PTT agents when irradiated with a near-infrared (NIR-II) laser. The use of an NIR-II laser enabled deeper penetration and higher maximum permissible exposure, which increased the photothermal efficacy, especially in humans in the clinic. The in vitro performance was demonstrated when irradiation of a 200 ppm Bi NP solution with a 1064-nm laser produced a hyperthermic effect, with a temperature increase in approximately 17.2 °C, in comparison to that of pure water, which caused a temperature increase in only approximately 2.8 °C. An in vitro clonogenic survival assay demonstrated the ability of the Bi@LyP-1 NPs to enhance the RT efficacy, even at low concentrations and low doses of radiation. The fraction of the 4T1 cells incubated in a 10 ppm Bi@LyP-1 NP solution that survived was dependent on X-ray irradiation doses. The combined PTT and RT effects were studied in vivo after Bi@LyP-1 NPs were IV injected. The surface temperature of the tumours after laser irradiation of 0.6 W cm−2 with a 1064-nm laser was monitored by NIR imaging. After Bi@LyP-1 NP injection, the tumour temperature rapidly increased to approximately 45 °C and then stabilized, while after a PBS injection, the temperature was found to increase slowly and only to 39.5 °C. This mild photothermal effect resulted only in a delay in tumour growth. However, after irradiation by X-ray and laser irradiation, tumour growth was completely inhibited, demonstrating strong synergistic effects.
4.2.3. CT/PA Imaging and PTT
Multifunctional Bi NPs were developed as theranostic agents for PA/CT imaging and NIR-PTT [23]. The preparation of 1,2-dilauroyl-sn-glycero-3 phosphocholine (DLPC)-coated spherical Bi NPs (Bi@DLPC NPs) was described, and these Bi NPs were characterized by a TEM-determined size of 47 nm and a hydrodynamic size of 162 nm. The photothermal effect was demonstrated in vitro. The temperature of a Bi@DLPC NP (500 μg mL−1) aqueous solution increased by 37 °C after 10 min of irradiation with an NIR laser (880 nm, power density of 1 W cm2). This kind of irradiation also induced mitochondrial damage by hyperthermia and MDA-MB-231 cell death in vitro, which was likely due to changes in cell membrane permeability.
PA and CT imaging were performed in vitro and in vivo. In vitro, the PA signal at 880 nm increased linearly with the Bi@DLPC NP concentration. A peak PA signal was observed in the MDA-MB-231 tumour cell-bearing mice 6 h after the intravenous injection of the Bi@DLPC NPs. In vitro, the CT signal intensity also linearly increased with the Bi@DLPC NP concentration.
The CT signal was observed in vivo after intratumoural injection of Bi@DLPC NPs (100 μL, 4 mg mL−1) into MDA-MB-231 tumour cell-bearing mice and PTT. Then, an in vivo photothermal effect was demonstrated with MDA-MB-231 tumour cell-bearing mice intravenously injected with Bi@DLPC NPs (1 mg mL−1) and irradiated with an NIR laser (880 nm, 1 W cm2) for 10 min. The thermic infrared images demonstrated that the surface temperature of the tumour area dramatically increased, from 37.4 to 57.7 °C. Cancer cell growth was abrogated by hyperthermia 14 days after treatment.
Physiologically stable PEG-modified polypyrrole-coated Bi Nanohybrids (Bi@PPy-PEG NHs) were recently prepared by Yang Sisi et al. [22]. The Bi@PPy-PEG NH aqueous dispersion revealed a high photothermal conversion capacity and stability after five repeated irradiation cycles with an 808-nm laser (2.0 W cm−2) completed within 10 min. The high efficiency was attributed to the contribution of PPy, which is known for its excellent photothermal effect. Consequently, highly effective photothermal ablation of cancer cells (4T1 cells) was achieved in vitro. At a concentration of 100 μg mL−1, the Bi@PPy-PEG NHs killed more than 90% of the 4T1 cells in 3 min after NIR 808 nm laser irradiation (2.0 W cm−2). Then, the PTT effect was determined in vivo with tumour-bearing BALB/c mice subjected to NIR 808-nm laser irradiation (2.0 W cm−2) twenty-four hours after the Bi@PPy-PEG NHs were IV injected.
Follow-up tumour volume measurements demonstrated that tumour growth was efficiently inhibited after irradiation. Furthermore, high-contrast CT/PA dual-modal imaging was demonstrated both in vitro and in vivo (intratumoural injection of Bi@PPy-PEG NHs in tumour-bearing BALB/c mice), showing great potential to provide accurate diagnostic information for antitumour treatment.
Lu et al. recently prepared Bi NPs coated with PEG-2000-2-distearoyl-sn-glycero-3-phosphoethanolamine (Bi@DSPE-PEG NPs), which were used as CT and PAI contrast agents and PTT agents [32]. The performance of the Bi@DSPE-PEG NPs as CT and PAI contrast agents was demonstrated with phantoms (HU measurement and PA signals as functions of Bi concentration) and in vivo by measurement of PA and CT signals after the Bi NPs were IV injected into the C6 glioma tumour-bearing mice. Photothermal therapeutic performance was demonstrated. The temperature of the Bi@DSPE-PEG NP solution (1 mg mL−1) rapidly increased to 70 °C within 4 min under 808-nm laser irradiation at a power density of 1.0 W cm−2. The same laser irradiation conditions were used to confirm this photothermal in vivo with C6 glioma tumour-bearing mice with Bi@DSPE-PEG NPs (200 μL of 10 mg mL−1 Bi NPs IV injected). Indeed, the tumour volume was significantly reduced 12 days after this PTT treatment.
4.2.4. CT/IRT Imaging and Chemo-Photothermal Therapy (CPTT)
Several Bi NPs have been developed with coatings containing natural biomolecules such as gelatin GEL (BGPs) [33]. These Bi NPs showed CT/infrared thermal (IRT) imaging capabilities as well as antitumor effects under CPTT in vitro and in vivo. After 15 min irradiation with an 808-nm laser (1.3 W cm−2), a strong increase in temperature is observed, up to 78.6 °C for a BGP suspension of 500 µg mL−1, showing the significant hyperthermia induced by Bi NPs compared to a deionized water solution. After five cycles of on–off irradiation, BGPs demonstrated an excellent photostability. The PTT effect was evaluated in vitro after 5 min irradiation on HepG-2 and HeLa cells with different BGP concentrations. Cell viability decreased below 10% with 500 µg mL−1 of BGPs after irradiation while it remained almost unchanged (90%) without irradiation. CT and IRT imaging were then performed on tumour-bearing mice, after IV injection of BGPs (20 mg kg−1) and exposure to NIR laser (808 nm, 1.3 W cm−2) for 5 min. CT imaging demonstrated that BGPs were excellent contrast agents, while IRT imaging measured a rapid temperature rise to 58 °C, demonstrating a favourable photothermal efficiency of BGPs on HepG-2 tumour cells. In vivo, after 24 h of IV injection of a mixture of BGPs and doxorubicin and under NIR laser irradiation, a significant growth inhibition of HepG-2 tumour cells was observed.
4.2.5. CT/PA/IRT Imaging and PTT
Pegylated metallic Bi NPs (Bi@PEG) were used to produce an in vitro and in vivo proof of concept of their use in trimodal imaging (CT, PA and infrared thermal (IRT)) and antitumour PTT [20].
The performance of the Bi NPs in CT imaging was demonstrated in vitro (HU measurements) and in vivo after they were IV injected into HeLa tumour cell-bearing BALB/c mice. After injection, the signal in the tumour increased gradually and considerably, with a burst 6 h post-injection. A linear PA signal was observed with the increasing aqueous concentration of the Bi@PEG NPs, indicating adequate PA imaging ability of the Bi NPs in vitro. PA imaging was investigated in vivo on tumour-bearing Balb/c mice after Bi NPs were IV injected. The PA signal in the tumour was enhanced and lasted up to 24 h, with the strongest signal observed 6 h after injection. The high imaging contrast and favourable residence time in the tumours make Bi NPs excellent contrast agents for both CT and PA imaging in vivo.
The in vitro PTT effect was confirmed by measuring the number of HeLa cancer cells that died after being incubated with Bi@PEG NPs and irradiated with a laser (808 nm, 1.0 W cm−2) for 10 min. The majority of the cells were destroyed under these conditions. The in vivo antitumour PTT was investigated in the HeLa tumour-bearing BALB/c mice after the Bi@PEG NPs were IV injected, and they were subjected to irradiation (808 nm, 1.0 W cm−2). During irradiation, real-time IRT imaging was used to monitor the tumour temperature change at different times. With laser irradiation, the tumour temperature rapidly increased and reached a plateau of ~51.6 °C. This temperature was sufficient to inhibit further tumour growth and to ablate the tumour after 10 days.
4.2.6. CT/MRI Imaging and Chemo/Photothermal/Chemodynamic Therapy
Stable Bi nanoparticles (NPs) coated by mesoporous silica (Bi@mSiO2@MnO2/DOX) were synthetized using a stepwise reaction protocol and a further loading with doxorubicin (DOX) [34]. In vitro, after laser irradiation (808 nm, power density, 1 W cm−2), Bi@mSiO2@MnO2 reached a stable photothermal effect with a high photothermal conversion efficiency of 50%. It was also demonstrated in vitro that MnO2 incorporated in the coating of these Bi NPs was able to catalyse H2O2 decomposition in O2. These Bi NPs are also efficient contrast agents for X-ray CT imaging of tumours with a high CT value (6.865 HU mM−1). The authors propose a sequential and synergistic mechanism of tumor cell destruction. First, after IV injection, Bi@mSiO2@MnO2/DOX accumulate in the tumour site. (NIR) light irradiation of Bi NPs induces hyperthermia and a PTT effect. The generated heat triggers the release of DOX and induces a chemotherapeutic effect. The chemotherapeutic effect is then enhanced due to the generation of O2 by decomposition of endogeneous H2O2 by MnO2. This production of O2 consumes glutathione to produce Mn2+, which provide a magnetic resonance imaging signal. Moreover, the presence of H2O2 and Mn2+ in the tumor site also produce toxic hydroxyl radicals, inducing a chemodynamic therapy effect. This clever and rational design of a theranostic Bi NP results in an excellent global performance, as Bi@mSiO2@MnO2/DOX-injected mice group treated with laser irradiation for 10 min exhibited a complete tumour elimination after 16 days, without any recurrence.
All these examples show the significant potential of Bi NPs to create versatile multifunctional theranostic nanomedicines. It is possible to design nanoparticles that are visible to several complementary imaging technologies in terms of spatial and temporal resolution and contrast. It is also possible to combine several types of synergistic treatment, in particular in oncology. However, the design of these nanoparticles remains complex and will require significant efforts to make their synthesis reliable in order to comply with cGMP drug manufacturing standards.
