Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by xueling xu and Version 2 by Jason Zhu.
Resveratrol, a natural polyphenolic stilbenoid, is a phytoestrogen. Most notably, accumulative evidence supports the notion that resveratrol has immunomodulatory and anticancer properties, and its antioxidant activity and ability to inhibit enzymes may contribute to its anti-inflammatory properties.
- resveratrol
- ovarian cancer
- antitumor
1. Introduction of Resveratrol
1.1. Sources
Resveratrol, 3,5,4′-trihydroxystilbene (Figure 1) was originally isolated in 1940 as an ingredient of white hellebore roots but was subsequently found in a variety of plants such as grapes, berries (i.e., blueberries, blackberries, cranberries and mulberries), peanuts, pines, eucalyptus, and rhubarb [1][14]. It is classified as a phytoalexin anti-fungicide with disease resistance in the plant kingdom. The accumulation of resveratrol in plants is due to the plant’s resistance mechanisms to parasites and other adverse conditions such as fungal infections, ultraviolet radiation, chemicals [2][15].
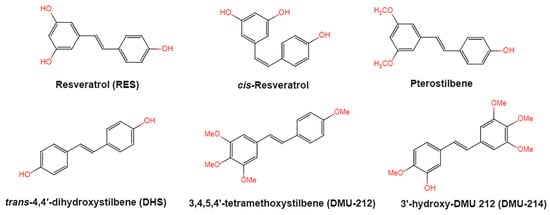
Figure 1. Structures of resveratrol, cis-Resveratrol, pterostilbene, trans-4,4′-dihydroxystilbene (DHS), 3,4,5,4′-tetramethoxystilbene (DMU-212), and 3′-hydroxy-3,4,5,4′-tetramethoxystilbene (DMU-214).
Concentrations of resveratrol vary from substance to substance. The concentration of resveratrol in blueberries is only 32 ng/g. Resveratrol concentrations in red and white wines are between 14 and 0.1 mg/L, respectively [3][16]. Concordantly, resveratrol concentrations in grape juice and whole grapes range from 0.05–0.5 mg/L and up to 3.54 mg/L [4][10]. The chemoprotective effects of resveratrol against cancer were first reported in 1997 and can be used to inhibit the tumogenesis in skin cancer [5][17]. Since then, resveratrol as an anticancer agent has been promoted to further research. Resveratrol has been indicated to be able to inhibit the proliferation of several types of cancer cells, including lymphoid and myeloid cancers; cancers of the breast, prostate, stomach, colon; melanoma; ovarian carcinoma; cervical carcinoma, and so on. Interestingly, researchers have discovered that high doses of resveratrol significantly extend lifespan in mammals. In addition, resveratrol can remove reactive oxygen species (ROS) and repress cyclooxygenase-1 (COX-1) or cyclooxygenase-2 (COX-2).
1.2. Chemistry of Resveratrol
Resveratrol is structurally based on stilbene and consists of two phenolic rings linked by styrene double bonds to form 3,4′,5-trihydroxystilbene, which exists in both the trans- and cis-iso forms. The trans-isoform is the dominant subtype and represents the most widely studied chemical form. Trans-resveratrol can undergo the cis-resveratrol form isomerization when exposed to solar or artificial light or ultraviolet radiation [6][7][8][18,19,20].
The structural modification of resveratrol has attracted the special attention of researchers, and many resveratrol derivatives, such as methoxylated, hydroxylated and halogenated derivatives, have been synthesized, showing favorable therapeutic potential [9][21]. Resveratrol is present in glycosylated forms in dietary products, called piceid, and maintains its biological effects and improves its overall stability and bioavailability [10][22]. In addition, since intestinal cells can only absorb the aglycone form of resveratrol, the absorption process requires glycosidase. Therefore, the relative content of the glycoside ligand and glycosylated resveratrol in food and beverage may regulate its absorption rate [11][23].
1.3. Absorption and Metabolism of Resveratrol
In the human intestines, 70–80% of resveratrol is rapidly absorbed by passive diffusion, while some is absorbed by forming complexes with membrane transporters such as integrins (Table 1) [12][13][24,25]. Once in the bloodstream, resveratrol can be found in three different forms: glucosidase, sulfate, or free form. In order to determine the concentration of resveratrol that can be achieved in human tissues after oral administration, patients with colorectal cancer were given 0.5–1.0 g of resveratrol once per day, and the levels of resveratrol and its metabolite, resveratrol-3-O-glucuronide, were recovered from tissues at high concentrations of 674 and 86.0 nmol/g, respectively [14][26]. Alternatively, circulating levels of trans-resveratrol accounted for 1.7 to 1.9% of the peak serum concentrations of total free resveratrol and conjugates in healthy males after a single oral dose of 25 mg/70 kg body weight [15][27].
Table 1.
Human oral administration of resveratrol metabolites in different liquids and tissues.
| Structure | Metabolite | Location | Reference | ||
|---|---|---|---|---|---|
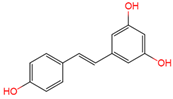 |
trans | -resveratrol | serum, plasma, urine | [12][16][17] | [24,28,29] |
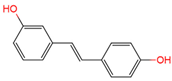 |
3,4′-O-dihydroxy-trans-stilbenes | urine | [17] | [29] | |
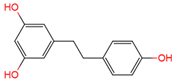 |
dihydroresveratrol | urine, plasma | [17][18] | [29,30] | |
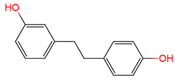 |
lunularin | urine | [17] | [29] | |
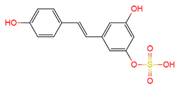 |
trans | -resveratrol-3-O-sulfate | plasma, urine | [12][19] | [24,31] |
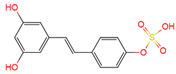 |
trans | -resveratrol-4′-O-sulfate | plasma, urine | [12][19] | [24,31] |
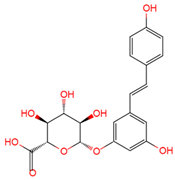 |
trans | -resveratrol-3-O-glucuronide | serum, plasma, urine | [12][16][19] | [24,28,31] |
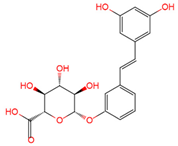 |
trans | -resveratrol-4′-O-glucuronide | serum, plasma, urine | [16][19] | [28,31] |
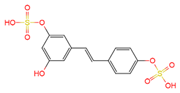 |
trans | -resveratrol-3,4′-O-disulfate | plasma | [19] | [31] |
Although in vitro studies have shown resveratrol to be highly potent in terms of its biologically beneficial effects in cells, its distribution in tissues is very low. This is due to resveratrol having a high metabolism, so its levels are very low. Many different metabolites are present in urine, such as resveratrol monosulfate, monosulfate dihydroresveratrol, and monoglucuronide dihydroresveratrol (Table 1) [28][40].
After ingestion, resveratrol travels to the intestine and then through the hepatic portal system to the liver, where it is metabolized. An initial concentration of resveratrol was detected in the blood 30 min after the oral administration of red wine to rats, and the concentration peaked after 60 min [29][30][41,42]. Tissue concentrations showed significant cardiac bioavailability and strong affinity for the liver and kidneys.
1.4. Bioavailability of Resveratrol
The limited bioavailability of resveratrol restricts its application and translatability. Possible methods to improve resveratrol bioavailability are developed resveratrol analogues and formulations such as adjuvants, nanoparticles, liposomes, micelles, and phospholipid complexes. In addition, several other approaches have been used to improve its bioavailability, including altering the route of resveratrol administration and blocking metabolic pathways by co-therapy with other drugs. Increasing the bioavailability of resveratrol increases its anti-tumor activity.
The exploitation of biopolymeric nanoparticles in recent years has improved the efficacy of anti-cancer drugs. The advantages of nanoparticle carriers are that they can target tumors, are more stable, and polyphenols can bind to the particles in a variety of ways, such as directly attaching themselves to the surface, becoming co-encapsulated with other compounds, or incorporating themselves within the surface of nanoparticles. As for the intraperitoneal injection of resveratrol, bovine serum albumin nanoparticles in ovarian cancer nude mice models showed higher concentrations of resveratrol in the blood and increased distribution in tissues such as liver, heart, kidney, and ovary [31][43]. Moreover, resveratrol–bovine serum albumin nanoparticles trigger human ovarian cancer cell line apoptosis by activating caspase [32][44].
Limited data in humans have demonstrated that resveratrol is quite pharmacologically safe. Currently, structural analogues of resveratrol with improved bioavailability are being sought as potential therapeutic cancer agents.
2. Molecular Mechanisms of Resveratrol Related to Ovarian Cancer
The influence of resveratrol on the cytochrome P450 (CYP) enzyme and cellular redox balance; the inhibition of estrogen hormone signaling; antiangiogenic and anti-inflammatory functions may all be related to its effects during the late stages of carcinogenesis.
2.1. Inhibition of Carcinogen Activation
Aromatase is a member of the CYP enzyme family and is encoded by the CYP19A1 gene [33][45]. In vivo, resveratrol blocks the activation of carcinogens via inhibiting the expression and activity of CYP1A1 that is induced by aryl hydrocarbon (Ah).
The present review sheds light on possible mechanisms by which resveratrol targets the aryl hydrocarbon receptor (AhR). It appears that this is possible by blocking the conversion of ligand-bound cytoplasmic AhR into its nuclear DNA-binding form or repressing the interaction of AhR with the transcription initiation complex on the promoter of CYP1A1 gene. Chen et al. concluded that resveratrol strongly inhibited the 2,3,7,8-tetrachlorodibenzo-p-dioxin-(TCDD)-induced AhR DNA binding activity as well as the transcription and catalytic activities of CYP1A1 and CYP1B1 in human mammary epithelial (MCF-10A) cells [34][46]. Furthermore, resveratrol notably reduced aromatase mRNA and protein abundance in SKBR-3 cells in a dose-dependent manner, suggesting that the compound could repress the transcriptional control dictated by promoter regulation [35][47].
2.2. Estrogen Effect
Estrogen stimulates ovarian cell proliferation and increases the metastatic potential of human ovarian cancer cell lines. The overexpression of estrogen receptor proteins has been described in more than two thirds of ovarian cancer cases [36][48]. Anti-estrogen therapy is one of the treatments for ovarian cancer. Understanding estrogen signaling response is essential to maximize the efficacy of anti-estrogen therapy for ovarian cancer. Studies have indicated that many antiestrogen-treated tumors maintain ER expression during relapse but that signaling through the ER pathway is altered in these resistant tumors. Therefore, targeting this pathway with resveratrol may influence the development of primary and secondary cancers.
In 1997, Gehm et al. demonstrated that resveratrol is a phytoestrogen [37][49]. In fact, resveratrol binds to the alpha and beta estrogen receptors (ERα and ERβ) with an affinity that is 7000 times stronger than estradiol [38][50]. Molecular dynamics studies have demonstrated that the binding of resveratrol to ERα is stereoselective, that is, the trans-isomer has a higher affinity for this receptor than the cis-isomer [39][51]. In particular, resveratrol appears to reduce estrogen signaling in the presence of ERα and ERβ, but in advanced cancer cells lacking ERβ, it is involved in tumor development.
Resveratrol can bind to estrogen receptors and can activate the transcription of antioxidant genes at concentrations that are similar to those required for its other biological effects. Resveratrol may function as a mixed estrogen agonist/antagonist in the absence of E2 but has been shown to exert antiestrogen activities in the presence of E2. Regarding its estrogenic activity, Zhang found that resveratrol-induced p53-dependent p21 gene expression and apoptosis are blocked by E2 in MCF-7 cells [40][52].
2.3. Antioxidant and Pro-Oxidant Effects
Resveratrol is an effective antioxidant that prevents the access of the oxidizing species to the lipids and scavenges free radicals before they can penetrate membrane. The antioxidant activity of resveratrol depends on the arrangement of functional groups on its nuclear structure. There is evidence indicating that the antioxidant effects of resveratrol are associated with the presence of hydroxyl groups, which are involved in the mechanisms of reducing ROS and free radicals and increasing endogenous antioxidant biosynthesis. At low doses, resveratrol interacts with the surface polar groups while localizing in the outer leaflet of the lipid bilayer at higher doses. The antioxidant activities of resveratrol are predominantly involved in the scavenging of ROS and reactive nitrogen species (RNS) and promoting the activity of a variety of antioxidant enzymes, such as superoxide dismutases (SODs), catalase, and glutathione peroxidase (GPX) [41][53]. Resveratrol can enhance gastrointestinal GPX promoter activity in HepG2 cells [42][54]. The antioxidant properties of resveratrol can also be attributed to its ability to decrease copper-mediated oxidation and the prevention of LDL and cell membranes lipid peroxidation [43][44][45][55,56,57].
It has been reported that the antioxidant properties of resveratrol have been successfully used to protect cells against hydrogen peroxide induced by oxidative stress and that pretreatment with resveratrol promotes cell survival and prevents cell death induced by ultraviolet radiation. Additionally, resveratrol increased SOD and GPX activity and retarded malondialdehyde levels in senescence-accelerated mice models at different doses given over 8 weeks [46][58].
In practice, every antioxidant is a redox agent, and resveratrol is no exception. It also undergoes an autooxidation process, leading to the production of H2O2 and complex mixtures of semiquinones and quinones. Copper is the most redox-active metal that exists in the nucleus, serum, and tissues [47][59]. Approximately 20% of copper is stored in the nucleus and binds with DNA both at intrastrand and interstrand levels [48][60]. Resveratrol possesses pro-oxidation properties that lead to the oxidative breakage of cellular DNA in the presence of transition metal ions such as copper [49][61]. In recent years, research has suggested that the pro-oxidative potential may be a shared mechanism for the anticancer and chemoprophylaxis characteristics of polyphenols. Copper ions are reported to be increased in various malignancies, so the present study might explain the anticancer activity of resveratrol in various cancer cell lines [50][62].
2.4. Inhibition of Angiogenesis
Angiogenesis is an important agent of tumor development. Sustained expansion of a tumor mass requires new blood vessel formation to provide rapidly multiplying tumor cells with sufficient oxygen and metabolites. However, because rapidly proliferating cells increase metabolic activity and oxygen consumption, tumors may maintain an intratumoral hypoxic environment [51][63]. The key regulator of hypoxia-induced angiogenesis is the transcription factor hypoxia inducible factor (HIF-1). Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), VEGF receptor (VEGFR), IL-8, inducible nitric oxide synthase (iNOS), and angiopoietin have been identified as HIF-regulated angiogenic factors [52][64].
A large number of studies have shown that resveratrol has anti-angiogenic effects. Resveratrol substantially induced HIF-1α protein degradation through the proteasome pathway and also greatly inhibited VEGF expression and thus provided a novel potential mechanism for inhibiting human ovarian cancer progression [53][65]. Furthermore, Garvin et al. observed notably lower tumor growth, significant increases in apoptosis, and decreased angiogenesis in ERα- ERβ+ MDA-MB-231 breast tumors in resveratrol-treated nude mice [54][66].
2.5. Anti-Inflammatory Effects of Resveratrol
Inflammation is a major driver of carcinogenesis, acting at all stages of tumorigenesis [55][56][67,68]. Components of inflammatory pathways, including free radicals, cytokines, nuclear transcription factor-kappa B (NF-κB), signal transduction and transcriptional activator 3 (STAT3), iNOS, COX-2, prostaglandin, and VEGF, have been proven to be associated with the development of numerous malignant tumors, including ovarian cancer. In ovarian cancer samples, COX-2 was found to be highly expressed in non-mucinous ovarian cancer, and COX-2 expression was significantly associated with adverse prognostic factors, such as stage, tumor grade, residual disease status, and the presence of ascites [57][69].
Previous hypotheses regarding the causes of ovarian cancer have attributed risk to an excess number of lifetime ovulations or to elevations in steroid hormones. Additionally, inflammation may underlie ovulatory events because an inflammatory reaction is induced during the process of ovulation [58][59][70,71]. Components of the inflammatory pathway include STAT3, NF-κB, iNOS, COX-2, and VEGF [60][72]. In addition, in a population-based, case–control study, the long-term use of nonsteroidal anti-inflammatory drugs (NSAID) was negatively associated with ovarian cancer risk [61][73].
Phytochemicals play an anticancer role by regulating various signaling pathways, one of which is inflammatory signaling. The inhibitory effects on tumor growth that are provided by resveratrol are, in part, mediated through its anti-inflammatory activity. A lot of their molecular targets occur on iNOS, COXs, leukotrienes, NF-κB, tumor necrosis factor-alpha (TNF-α), interleukins (ILs), etc. [62][74]. In murine and human macrophage cells, resveratrol blocks the TNF-induced activation of NF-κB in a dose- and time-dependent manner [63][64][75,76]. Resveratrol also inhibits reactive oxygen intermediate generation and lipid peroxidation induced by TNF.
Many other inflammation-related proteins, such as AKT (i.e., protein kinase B), lysophosphatidic acid (LPA), and protein kinase C (PKC) are also overexpressed in more than 70% of ovarian cancers [65][77]. Interestingly, LPA has also been found to cause the upregulation of IL-6, IL-8, and VEGF in epithelial ovarian cancer (EOC) cell lines through the Gi/phosphoinositide 3-kinase (PI3K)-AKT/NF-κB pathway [66][78]. In ovarian cancer cells, resveratrol inhibits the expression of HIF-1α. The underlying mechanism of inhibition appears to be responsible for both the inactivation of mitogen-activated protein kinase (MAPK) and p70S6K, resulting in a profound decrease in VEGF expression and cell migration [67][79]. Collectively, these data suggest that resveratrol may inhibit ovarian carcinogenesis by downregulating NF-κB activity and blocking HIF-1α and VEGF expression. The regulation of mal-regulated inflammatory pathways is a promising chemoprophylaxis strategy against ovarian cancer.
