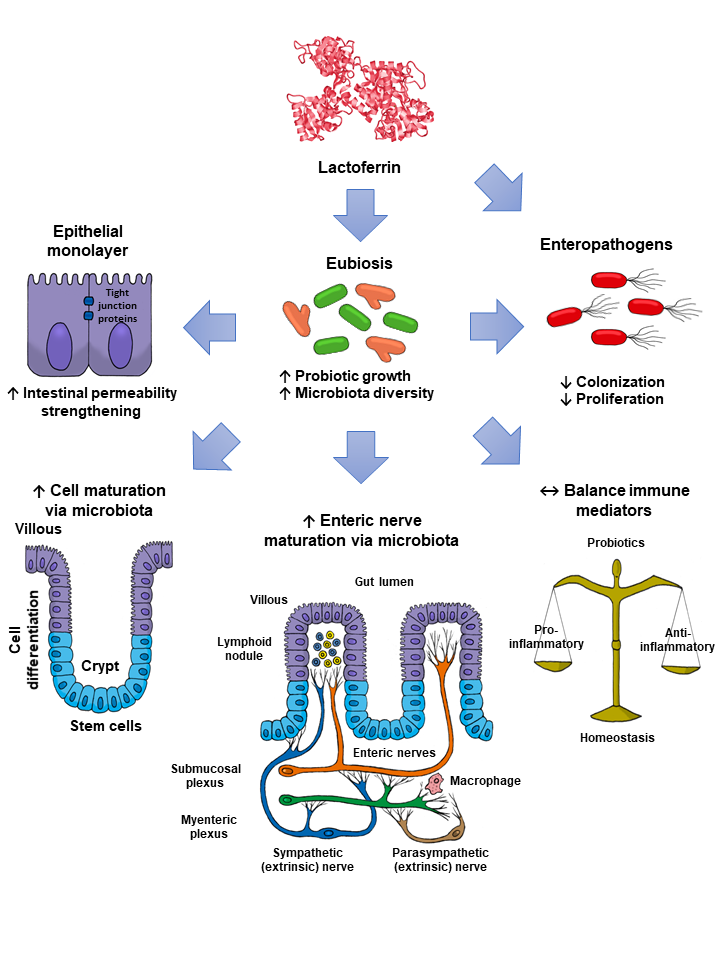
Lactoferrin (Lf) is an iron-binding milk glycoprotein that promotes the growth of selected probiotic strains. The effect of Lf on the growth and diversification of intestinal microbiota may have an impact on several issues, including (i) strengthening the permeability of the epithelial cell monolayer, (ii) favoring the microbial antagonism that discourages the colonization and proliferation of enteric pathogens, (iii) enhancing the growth and maturation of cell-monolayer components and gut nerve fibers, and (iv) providing signals to balance the anti- and pro-inflammatory responses resulting in gut homeostasis.
- lactoferrin
- lactoferricin
- milk
1. Introduction
2. Lactoferrin: Effects on Probiotic Growth
2.1. Bulk Milk
2.2. Apo and HoloLactoferrin
2.3. Probiotic Culture Conditions
2.4. Probiotic Lactoferrin-Binding Proteins
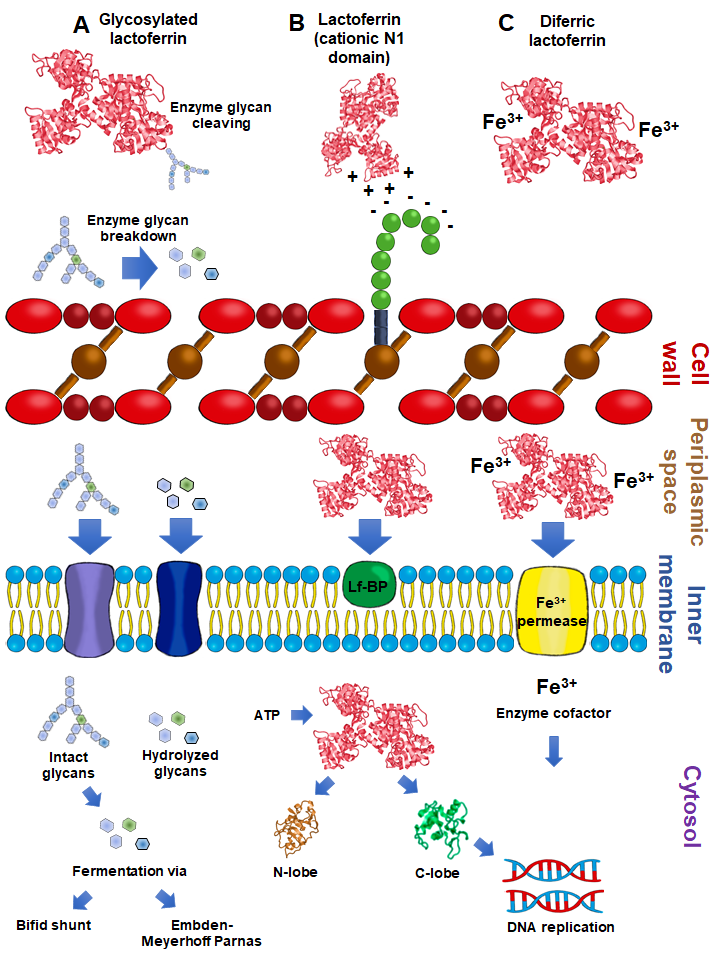
2.5. Lactoferrin Hydrolysates and Lf-Derived Peptides
3. Lactoferrin: Modulatory Effects on the Gut Microbiota
3.1. Human neonates
3.2. Piglet Model
By contrast, piglets fed the chimera Lfcin-Lfampin showed enhanced serum antioxidant enzymes such as glutathione peroxidase and peroxidase, as well as the effectors of the adaptive immunity branch including IgA, IgG, and IgM antibodies and the components of the innate response involved in protection from the deleterious effects of inflammation [61]. This finding agrees with the observation that rhLf-cow milk increased TLR-2 mRNA expression in the ileum and the levels of colonic IgG and nuclear factor-κB (NF-κB) p65, concomitant with the increase in spleen IL-2, -4, and -5 and plasmatic IgG, IgA and IL-12, and IL-10 [69]. Although the assessment of parameters was systemic, the compartmental modulatory action on inflammatory and immune components underlies the protective role of recombinant Lf derivatives in the intestinal milieu. According to the above results, some presumable mechanisms that account for the impact of Lf on intestinal microbiota growth are depicted in Figure 2.
References
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61.
- Nazir, S.N.M.; Yasmeen, A.; Usman, S. Review study on lactoferrin: A multifunctional protein. Sky J. Food Sci. 2017, 6, 014–020.
- Albar, A.H.; Almehdar, H.A.; Uversky, V.N.; Redwan, E.M. Structural heterogeneity and multifunctionality of lactoferrin. Curr. Protein Pept. Sci. 2014, 15, 778–797.
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840.
- Petschow, B.W.; Talbott, R.D. Response of bifidobacterium species to growth promoters in human and cow milk. Pediatric Res. 1991, 29, 208–213.
- Sela, D.A.; Mills, D.A. Nursing our microbiota: Molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010, 18, 298–307.
- Loh, S., Jr.; Maznah, I. The effect of different milks and milk proteins on the growth of Bifidobacterium infantis ATCC 27920 in vitro. Malays. J. Nutr. 1999, 5, 61–70.
- Petschow, B.W.; Talbott, R.D. Growth promotion of Bifidobacterium species by whey and casein fractions from human and bovine milk. J. Clin. Microbiol. 1990, 28, 287–292.
- Kodama, A. The biotying of Bifidobacterium isolated from healthy infants in Wakayama and Osaka district; Bifidobacterium growth promoting activities of human casein and lactoferrin. Pediatr Int. 1983, 25, 486.
- Petschow, B.W.; Talbott, R.D.; Batema, R.P. Ability of lactoferrin to promote the growth of Bifidobacterium spp. in vitro is independent of receptor binding capacity and iron saturation level. J. Med. Microbiol. 1999, 48, 541–549.
- Garrido, D.; Nwosu, C.; Ruiz-Moyano, S.; Aldredge, D.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Endo-beta-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol. Cell. Proteom. 2012, 11, 775–785.
- Karav, S.; German, J.B.; Rouquie, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870.
- Zhao, J.; Cheung, P.C. Comparative proteome analysis of Bifidobacterium longum subsp. infantis grown on beta-glucans from different sources and a model for their utilization. J. Agric. Food Chem. 2013, 61, 4360–4370.
- Leon-Sicairos, N.; Lopez-Soto, F.; Reyes-Lopez, M.; Godinez-Vargas, D.; Ordaz-Pichardo, C.; de la Garza, M. Amoebicidal activity of milk, apo-lactoferrin, sIgA and lysozyme. Clin. Med. Res. 2006, 4, 106–113.
- Griffiths, E.A.; Duffy, L.C.; Schanbacher, F.L.; Dryja, D.; Leavens, A.; Neiswander, R.L.; Qiao, H.; DiRienzo, D.; Ogra, P. In vitro growth responses of bifidobacteria and enteropathogens to bovine and human lactoferrin. Dig. Dis. Sci. 2003, 48, 1324–1332.
- Saito, H.; Miyakawa, H.; Ishibashi, N.; Yoshitaka, T.; Hayasawa, H.; Shimamura, S. Effect of Iron-Free and Metal-Bound Forms of Lactoferrin on the Growth of Bifidobacteria.; E. coli and S. aureus. Biosci. Microflora 1996, 15, 1–7.
- Kim, W.S.; Ohashi, M.; Tanaka, T.; Kumura, H.; Kim, G.Y.; Kwon, I.K.; Goh, J.S.; Shimazaki, K. Growth-promoting effects of lactoferrin on L. acidophilus and Bifidobacterium spp. Biometals 2004, 17, 279–283.
- Miller-Catchpole, R.; Kot, E.; Haloftis, G.; Furmanov, S.; Bezkorovainy, A. Lactoferrin can supply iron for the growth of Bifidobacterium breve. Nutr Res. 1997, 17, 205–213.
- Cronin, M.; Zomer, A.; Fitzgerald, G.F.; van Sinderen, D. Identification of iron-regulated genes of Bifidobacterium breve UCC2003 as a basis for controlled gene expression. Bioengineered 2012, 3, 157–167.
- Lanigan, N.; Bottacini, F.; Casey, P.G.; O’Connell Motherway, M.; van Sinderen, D. Genome-Wide Search for Genes Required for Bifidobacterial Growth under Iron-Limitation. Front. Microbiol. 2017, 8, 964.
- Chen, P.W.; Liu, Z.S.; Kuo, T.C.; Hsieh, M.C.; Li, Z.W. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. Biometals 2017, 30, 237–248.
- Chen, P.W.; Ku, Y.W.; Chu, F.Y. Influence of bovine lactoferrin on the growth of selected probiotic bacteria under aerobic conditions. Biometals 2014, 27, 905–914.
- Kim, W.S. Effects of Heat-treated Bovine Lactoferrin on the Growth of Lactococcus lactis subsp. cremoris JCM 20076. J. Milk Sci. Biotechnol. 2019, 37, 129–135.
- Kim, W.S.; Tanaka, T.; Kumura, H.; Shimazaki, K. Lactoferrin-binding proteins in Bifidobacterium bifidum. Biochem. Cell Biol. 2002, 80, 91–94.
- Wasko, A.; Polak-Berecka, M.; Kalita, M. Protein profiles from intact cells as a tool in Bifidobacterium characteristics. Pol. J. Microbiol. 2012, 61, 305–310.
- Rahman, M.M.; Kim, W.S.; Ito, T.; Kumura, H.; Shimazaki, K. Examination of bovine lactoferrin binding to bifidobacteria. Appl. Biochem. Microbiol. 2008, 44, 529–532.
- Rahman, M.M.; Kim, W.S.; Ito, T.; Kumura, H.; Shimazaki, K. Growth promotion and cell binding ability of bovine lactoferrin to Bifidobacterium longum. Anaerobe 2009, 15, 133–137.
- Rahman, M.; Kim, W.S.; Kumura, H.; Shimazaki, K. Bovine lactoferrin region responsible for binding to bifidobacterial cell surface proteins. Biotechnol. Lett. 2009, 31, 863–868.
- Semenov, D.V.; Kanyshkova, T.G.; Buneva, V.N.; Nevinsky, G.A. Human milk lactoferrin binds ATP and dissociates into monomers. Biochem. Mol. Biol. Int. 1999, 47, 177–184.
- Kanyshkova, T.G.; Babina, S.E.; Semenov, D.V.; Isaeva, N.; Vlassov, A.V.; Neustroev, K.N.; Kul’minskaya, A.A.; Buneva, V.N.; Nevinsky, G.A. Multiple enzymic activities of human milk lactoferrin. Eur. J. Biochem. 2003, 270, 3353–3361.
- He, J.; Furmanski, P. Sequence specificity and transcriptional activation in the binding of lactoferrin to DNA. Nature 1995, 373, 721–724.
- Vinderola, C.G.; Medici, M.; Perdigon, G. Relationship between interaction sites in the gut, hydrophobicity, mucosal immunomodulating capacities and cell wall protein profiles in indigenous and exogenous bacteria. J. Appl. Microbiol. 2004, 96, 230–243.
- Rahman, M.; Kim, W.S.; Kumura, H.; Shimazaki, K. In vitro effects of bovine lactoferrin on autoaggregation ability and surface hydrophobicity of bifidobacteria. Anaerobe 2008, 14, 73–77.
- Andriantsoanirina, V.; Teolis, A.C.; Xin, L.X.; Butel, M.J.; Aires, J. Bifidobacterium longum and Bifidobacterium breve isolates from preterm and full term neonates: Comparison of cell surface properties. Anaerobe 2014, 28, 212–215.
- Canzi, E.; Guglielmetti, S.; Mora, D.; Tamagnini, I.; Parini, C. Conditions affecting cell surface properties of human intestinal bifidobacteria. Antonie Van Leeuwenhoek 2005, 88, 207–219.
- Chen, P.W.; Jheng, T.T.; Shyu, C.L.; Mao, F.C. Antimicrobial potential for the combination of bovine lactoferrin or its hydrolysate with lactoferrin-resistant probiotics against foodborne pathogens. J. Dairy Sci. 2013, 96, 1438–1446.
- Chen, P.W.; Jheng, T.T.; Shyu, C.L.; Mao, F.C. Synergistic antibacterial efficacies of the combination of bovine lactoferrin or its hydrolysate with probiotic secretion in curbing the growth of meticillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2013, 62, 1845–1851.
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Sato, T.; Xiao, J.Z.; Abe, F.; Iwatsuki, K. Isolation of a bifidogenic peptide from the pepsin hydrolysate of bovine lactoferrin. Appl. Environ. Microbiol. 2013, 79, 1843–1849.
- Liepke, C.; Adermann, K.; Raida, M.; Magert, H.J.; Forssmann, W.G.; Zucht, H.D. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur. J. Biochem. 2002, 269, 712–718.
- Paul, M.; Somkuti, G.A. Hydrolytic breakdown of lactoferricin by lactic acid bacteria. J. Ind. Microbiol. Biotechnol. 2010, 37, 173–178.
- Kim, W.S.; Ohashi, M.; Shimazaki, K. Inhibitory Effects of Synthetic Peptides Containing Bovine Lactoferrin C-lobe Sequence on Bacterial Growth. Korean J. Food Sci. Anim. Resour. 2016, 36, 452–457.
- Goldman, A.S. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J. Nutr. 2000, 130, 426S–431S.
- Donovan, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatrics 2016, 173, S16–S28.
- Schuller, S.S.; Kramer, B.W.; Villamor, E.; Spittler, A.; Berger, A.; Levy, O. Immunomodulation to Prevent or Treat Neonatal Sepsis: Past, Present, and Future. Front. Pediatrics 2018, 6, 199.
- Buccigrossi, V.; de Marco, G.; Bruzzese, E.; Ombrato, L.; Bracale, I.; Polito, G.; Guarino, A. Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatr Res. 2007, 61, 410–414.
- Balmer, S.E.; Scott, P.H.; Wharton, B.A. Diet and fecal flora in the newborn: Lactoferrin. Arch. Dis. Child. 1989, 64, 1685–1690.
- Wharton, B.A.; Balmer, S.E.; Scott, P.H. Fecal flora in the newborn. Effect of lactoferrin and related nutrients. Adv. Exp. Med. Biol. 1994, 357, 91–98.
- Mastromarino, P.; Capobianco, D.; Campagna, G.; Laforgia, N.; Drimaco, P.; Dileone, A.; Baldassarre, M.E. Correlation between lactoferrin and beneficial microbiota in breast milk and infant’s feces. Biometals 2014, 27, 1077–1086.
- Sherman, M.P.; Sherman, J.; Arcinue, R.; Niklas, V. Randomized Control Trial of Human Recombinant Lactoferrin: A Substudy Reveals Effects on the Fecal Microbiome of Very Low Birth Weight Infants. J. Pediatrics 2016, 173, S37–S42.
- Roberts, A.K.; Chierici, R.; Sawatzki, G.; Hill, M.J.; Volpato, S.; Vigi, V. Supplementation of an adapted formula with bovine lactoferrin: 1. Effect on the infant fecal flora. Acta paediatrica 1992, 81, 119–124.
- Unger, S.; Stintzi, A.; Shah, P.; Mack, D.; O’Connor, D.L. Gut microbiota of the very-low-birth-weight infant. Pediatric Res. 2015, 77, 205–213.
- Meyer, M.P.; Alexander, T. Reduction in necrotizing enterocolitis and improved outcomes in preterm infants following routine supplementation with Lactobacillus GG in combination with bovine lactoferrin. J. Neonatal-Perinat. Med. 2017, 10, 249–255.
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr 2016, 173, S43–S52.
- Manzoni, P.; Mostert, M.; Stronati, M. Lactoferrin for prevention of neonatal infections. Curr. Opin. Infect. Dis 2011, 24, 177–182.
- Nguyen, D.N.; Li, Y.; Sangild, P.T.; Bering, S.B.; Chatterton, D.E. Effects of bovine lactoferrin on the immature porcine intestine. Br. J. Nutr. 2014, 111, 321–331.
- Nguyen, D.N.; Jiang, P.; Stensballe, A.; Bendixen, E.; Sangild, P.T.; Chatterton, D.E. Bovine lactoferrin regulates cell survival, apoptosis and inflammation in intestinal epithelial cells and preterm pig intestine. J. Proteom. 2016, 139, 95–102.
- Tang, X.S.; Shao, H.; Li, T.J.; Tang, Z.R.; Huang, R.L.; Wang, S.P.; Kong, X.F.; Wu, X.; Yin, Y.L. Dietary supplementation with bovine lactoferrampin-lactoferricin produced by Pichia pastoris fed-batch fermentation affects intestinal microflora in weaned piglets. Appl. Biochem. Biotechnol. 2012, 168, 887–898.
- Grzywacz, K.; Butcher, J.; Romain, G.; Li, J.; Stintzi, A. The impact of probiotics and lactoferrin supplementation on piglet gastrointestinal microbial communities. Biometals 2019, 32, 533–543.
- Berding, K.; Wang, M.; Monaco, M.H.; Alexander, L.S.; Mudd, A.T.; Chichlowski, M.; Waworuntu, R.V.; Berg, B.M.; Miller, M.J.; Dilger, R.N.; et al. Prebiotics and Bioactive Milk Fractions Affect Gut Development, Microbiota, and Neurotransmitter Expression in Piglets. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 688–697.
- Hu, W.; Zhao, J.; Wang, J.; Yu, T.; Wang, J.; Li, N. Transgenic milk containing recombinant human lactoferrin modulates the intestinal flora in piglets. Biochem. Cell Biol. 2012, 90, 485–496.
- Tang, Z.; Yin, Y.; Zhang, Y.; Huang, R.; Sun, Z.; Li, T.; Chu, W.; Kong, X.; Li, L.; Geng, M.; et al. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 2009, 101, 998–1005.
- Sherman, M.P.; Zaghouani, H.; Niklas, V. Gut microbiota, the immune system, and diet influence the neonatal gut-brain axis. Pediatric Res. 2015, 77, 127–135.
- Yang, C.; Zhu, X.; Liu, N.; Chen, Y.; Gan, H.; Troy, F.A.; Wang, B. Lactoferrin up-regulates intestinal gene expression of brain-derived neurotrophic factors BDNF, UCHL1 and alkaline phosphatase activity to alleviate early weaning diarrhea in postnatal piglets. J. Nutr. Biochem. 2014, 25, 834–842.
- Cooper, C.A.; Nelson, K.M.; Maga, E.A.; Murray, J.D. Consumption of transgenic cows’ milk containing human lactoferrin results in beneficial changes in the gastrointestinal tract and systemic health of young pigs. Transgenic Res. 2013, 22, 571–578.
- Reznikov, E.A.; Comstock, S.S.; Yi, C.; Contractor, N.; Donovan, S.M. Dietary bovine lactoferrin increases intestinal cell proliferation in neonatal piglets. J. Nutr. 2014, 144, 1401–1408.
- Qi, Z.; Chen, Y.G. Regulation of intestinal stem cell fate specification. Sci. China Life Sci. 2015, 58, 570–578.
- Jahan, M.; Kracht, S.; Ho, Y.; Haque, Z.; Bhattachatyya, B.N.; Wynn, P.C.; Wang, B. Dietary lactoferrin supplementation to gilts during gestation and lactation improves pig production and immunity. PloS ONE 2017, 12, e0185817.
- Comstock, S.S.; Reznikov, E.A.; Contractor, N.; Donovan, S.M. Dietary bovine lactoferrin alters mucosal and systemic immune cell responses in neonatal piglets. J. Nutr. 2014, 144, 525–532.
- Li, Q.; Hu, W.; Zhao, J.; Wang, J.; Dai, Y.; Zhao, Y.; Meng, Q.; Li, N. Supplementation transgenic cow’s milk containing recombinant human lactoferrin enhances systematic and intestinal immune responses in piglets. Mol. Biol. Rep. 2014, 41, 2119–2128.
