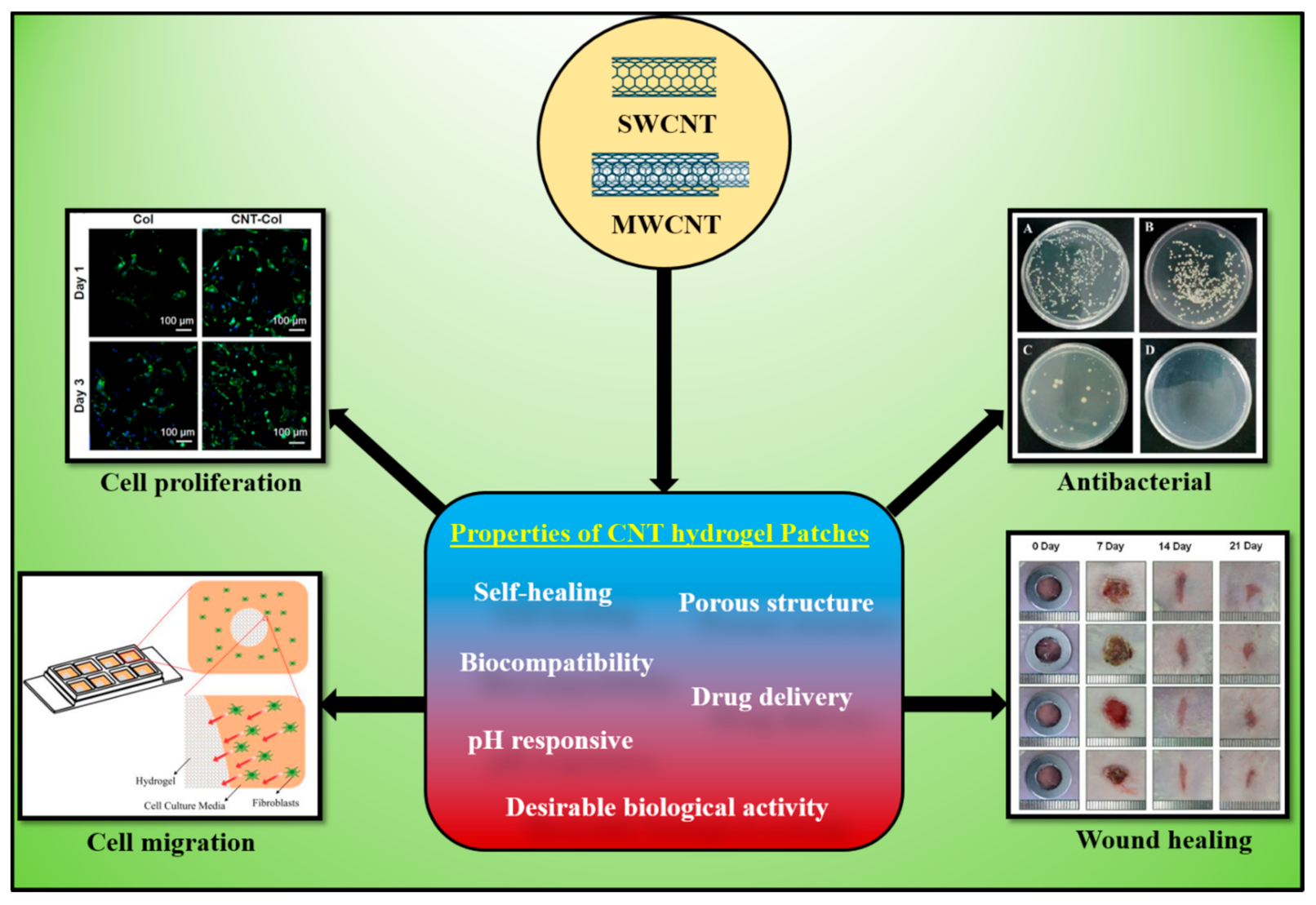
Carbonaceous materials, including carbon nanotubes (CNTs), have been widely explored in wound healing and other applications because of their superior physicochemical and potential biomedical properties to the nanoscale level. CNTs-based hydrogels are widely used for wound-healing and antibacterial applications. CNTs-based materials exhibited improved antimicrobial, antibacterial, adhesive, antioxidants, and mechanical properties, which are beneficial for the wound-healing process.
- carbon nanotubes (CNTs)
- conductive hydrogels
- antibacterial
- wound healing
1. Introduction
CNT Composite | Highlight | Biological Application | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
CP@CNT | Examine CNT as a nanocarrier of drug CP | Cyclophosphamide, anticancer drug delivery, reduced side effects |
[23] |
||||||||
CNT-Alg-Ch-FA | Penetration of functionalized CNT through cell membrane | Doxorubicin hydrochloride delivery for cancer treatment |
[24] |
||||||||
CNT-UHMWPE | CNT incorporation showed high mechanical and tribological properties | Sustained release (up to 429 h) of gentamicin |
[25] |
||||||||
CNT-3H2PO4 | Binding energy of drug to CNT increased with more H2PO4moeities | Delivery for anti-osteoporosis zolendronate and risedronate drugs |
[26] |
||||||||
PLGA-CNT-PDA-lam | PDA modified scaffold can adhere laminin for longer time and promote neurite outgrowth | Enhancement of PC12 cells for nerve tissue engineering |
[27] |
||||||||
Rh-CNT | Analysis of gases of lung cancer (C6H6 and C6H7N) | Biosensor for prediagnosis of lung cancer |
[28] |
2. Conductive Properties of CNTs
Properties | Units | SWCNT | MWCNT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Specific gravity (bulk) | g/cm3 | 0.8–1.3 | 1.8–2.6 | ||||||||
Specific area | m2/g | 400–900 | 200–400 | ||||||||
Young’s modulus | Pa | ≈1000 | ≈1000 | ||||||||
Tensile strength | Pa | 3.1010–5.1011 | 1.1010–15.1010 | ||||||||
Thermal conductivity | W/m.K | 3000–6000 | 2000–3000 | ||||||||
Electrical conductivity | S/cm | 102–106 | 103–105 | ||||||||
Thermal stability temperature in air | °C | 550–650 | 550–650 |
3. Development of CNT-Based Conductive Hydrogels
4. CNT-Based Antibacterial Applications
References
- Khalil, H.; Cullen, M.; Chambers, H.; Carroll, M.; Walker, J. Reduction in wound healing times, cost of consumables and number of visits treated through the implementation of an electronic wound care system in rural Australia. Int. Wound J. 2016, 13, 945–950.
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674.
- Feily, A.; Moeineddin, F.; Mehraban, S. Physical Modalities in the Management of Wound (s). In Wound Healing-New Insights into Ancient Challenges; IntechOpen: London, UK, 2016.
- Kosaric, N.; Kiwanuka, H.; Gurtner, G.C. Stem cell therapies for wound healing. Expert Opin. Biol. Ther. 2019, 19, 575–585.
- Gottrup, F.; Dissemond, J.; Baines, C.; Frykberg, R.; Jensen, P.Ø.; Kot, J.; Kröger, K.; Longobardi, P. Use of oxygen therapies in wound healing. J. Wound Care 2017, 26, S1–S43.
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric oxide therapy for diabetic wound healing. Adv. Healthc. Mater. 2019, 8, 1801210.
- Parani, M.; Lokhande, G.; Singh, A.; Gaharwar, A.K. Engineered nanomaterials for infection control and healing acute and chronic wounds. ACS Appl. Mater. Interfaces 2016, 8, 10049–10069.
- Borena, B.M.; Martens, A.; Broeckx, S.Y.; Meyer, E.; Chiers, K.; Duchateau, L.; Spaas, J.H. Regenerative skin wound healing in mammals: State-of-the-art on growth factor and stem cell based treatments. Cell. Physiol. Biochem. 2015, 36, 1–23.
- Pereira, R.F.; Bártolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care 2014, 5, 208–229.
- Vieira, S.; Castelli, S.; Falconi, M.; Takarada, J.; Fiorillo, G.; Buzzetti, F.; Lombardi, P.; Desideri, A. Role of 13-(di)phenylalkyl berberine derivatives in the modulation of the activity of human topoisomerase IB. Int. J. Biol. Macromol. 2015, 77, 68–75.
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-driven therapeutic interventions in wound healing: Potential uses and applications. ACS Cent. Sci. 2017, 3, 163–175.
- Korupalli, C.; Li, H.; Nguyen, N.; Mi, F.-L.; Chang, Y.; Lin, Y.-J.; Sung, H.-W. Conductive materials for healing wounds: Their incorporation in electroactive wound dressings, characterization, and perspectives. Adv. Healthc. Mater. 2021, 10, 2001384.
- Vashist, A.; Kaushik, A.; Vashist, A.; Sagar, V.; Ghosal, A.; Gupta, Y.K.; Ahmad, S.; Nair, M. Advances in carbon nanotubes-hydrogel hybrids in nanomedicine for therapeutics. Adv. Healthc. Mater. 2018, 7, e1701213.
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074.
- Xiang, C.; Zhang, Y.; Guo, W.; Liang, X.-J. Biomimetic carbon nanotubes for neurological disease therapeutics as inherent medication. Acta Pharm. Sin. B 2020, 10, 239–248.
- Saito, N.; Usui, Y.; Aoki, K.; Narita, N.; Shimizu, M.; Hara, K.; Ogiwara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Carbon nanotubes: Biomaterial applications. Chem. Soc. Rev. 2009, 38, 1897–1903.
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon nanotubes in biomedical applications: Factors, mechanisms, and remedies of toxicity. J. Med. Chem. 2016, 59, 8149–8167.
- Liu, Y.; Zhou, J.; Zhang, X.; Liu, Z.; Wan, X.; Tian, J.; Wang, T.; Chen, Y. Synthesis, characterization and optical limiting property of covalently oligothiophene-functionalized graphene material. Carbon 2009, 47, 3113–3121.
- Sinha, N.; Yeow, J.T. Carbon nanotubes for biomedical applications. IEEE Trans. NanoBiosci. 2005, 4, 180–195.
- Prajapati, S.K.; Malaiya, A.; Kesharwani, P.; Soni, D.; Jain, A. Biomedical applications and toxicities of carbon nanotubes. Drug Chem. Toxicol. 2020, 1–16.
- Adorinni, S.; Rozhin, P.; Marchesan, S. Smart hydrogels meet carbon nanomaterials for new frontiers in medicine. Biomedicines 2021, 9, 570.
- Asadi, N.; Pazoki-Toroudi, H.; Del Bakhshayesh, A.R.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750.
- Kakaei, A.; Mirzaei, M. CNT: In silico exploration of nano drug delivery system. Lab-in-Silico 2021, 2, 9–14.
- Sohrabi, N.; Alihosseini, A.; Pirouzfar, V.; Pedram, M.Z. Analysis of dynamics targeting CNT-based drug delivery through lung cancer cells: Design, simulation, and computational approach. Membranes 2020, 10, 283.
- Manoj Kumar, R.; Rajesh, K.; Haldar, S.; Gupta, P.; Murali, K.; Roy, P.; Lahiri, D. Surface modification of CNT reinforced UHMWPE composite for sustained drug delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 748–759.
- Nikfar, Z.; Shariatinia, Z. DFT computational study on the phosphate functionalized SWCNTs as efficient drug delivery systems for anti-osteoporosis zolendronate and risedronate drugs. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 91, 41–59.
- Nazeri, N.; Karimi, R.; Ghanbari, H. The effect of surface modification of poly-lactide-co-glycolide/carbon nanotube nanofibrous scaffolds by laminin protein on nerve tissue engineering. J. Biomed. Mater. Res. Part. A 2021, 109, 159–169.
- Wan, Q.; Xu, Y.; Chen, X.; Xiao, H. Exhaled gas detection by a novel Rh-doped CNT biosensor for prediagnosis of lung cancer: A DFT study. Mol. Phys. 2018, 116, 2205–2212.
- Zhang, X.; Qin, M.; Xu, M.; Miao, F.; Merzougui, C.; Zhang, X.; Wei, Y.; Chen, W.; Huang, D. The fabrication of antibacterial hydrogels for wound healing. Eur. Polym. J. 2021, 146, 110268.
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784.
- Sousa, M.P.; Neto, A.I.; Correia, T.R.; Miguel, S.P.; Matsusaki, M.; Correia, I.J.; Mano, J.F. Bioinspired multilayer membranes as potential adhesive patches for skin wound healing. Biomater. Sci. 2018, 6, 1962–1975.
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77.
- Tavakoli, J.; Mirzaei, S.; Tang, Y. Cost-effective double-layer hydrogel composites for wound dressing applications. Polymers 2018, 10, 305.
- Sun, H.; Mou, Y.; Li, Y.; Li, X.; Chen, Z.; Duval, K.; Huang, Z.; Dai, R.; Tang, L.; Tian, F. Carbon nanotube-based substrates promote cardiogenesis in brown adipose-derived stem cells via β1-integrin-dependent TGF-β1 signaling pathway. Int. J. Nanomed. 2016, 11, 4381.
- Ravanbakhsh, H.; Bao, G.; Mongeau, L. Carbon nanotubes promote cell migration in hydrogels. Sci. Rep. 2020, 10, 2543.
- Shi, H.; Liu, H.; Luan, S.; Shi, D.; Yan, S.; Liu, C.; Li, R.K.Y.; Yin, J. Effect of polyethylene glycol on the antibacterial properties of polyurethane/carbon nanotube electrospun nanofibers. RSC Adv. 2016, 6, 19238–19244.
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1.
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
- Ahmad, M.; Silva, S.R.P. Low temperature growth of carbon nanotubes—A review. Carbon 2020, 158, 24–44.
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447.
- Faraji, S.; Stano, K.L.; Yildiz, O.; Li, A.; Zhu, Y.; Bradford, P.D. Ultralight anisotropic foams from layered aligned carbon nanotube sheets. Nanoscale 2015, 7, 17038–17047.
- Lee, E.; Salgado, R.A.; Lee, B.; Sumant, A.V.; Rajh, T.; Johnson, C.; Balandin, A.A.; Shevchenko, E.V. Design of lithium cobalt oxide electrodes with high thermal conductivity and electrochemical performance using carbon nanotubes and diamond particles. Carbon 2018, 129, 702–710.
- Marconnet, A.M.; Panzer, M.A.; Goodson, K.E. Thermal conduction phenomena in carbon nanotubes and related nanostructured materials. Rev. Mod. Phys. 2013, 85, 1295–1326.
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944.
- Maruyama, S. A molecular dynamics simulation of heat conduction of a finite length single-walled carbon nanotube. Microscale Thermophys. Eng. 2003, 7, 41–50.
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks: A review. J. Mater. Sci. 2019, 54, 7397–7427.
- Berber, S.; Kwon, Y.-K.; Tománek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4613–4616.
- Osman, M.A.; Srivastava, D. Temperature dependence of the thermal conductivity of single-wall carbon nanotubes. Nanotechnology 2001, 12, 21.
- Cao, Q.; Yu, Q.; Connell, D.W.; Yu, G. Titania/carbon nanotube composite (TiO2/CNT) and its application for removal of organic pollutants. Clean Technol. Environ. Policy 2013, 15, 871–880.
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199.
- Zhang, M.; Chen, S.; Zhong, L.; Wang, B.; Wang, H.; Hong, F. Zn2+-loaded TOBC nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 143, 235–242.
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 2019, 15, 1900046.
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835.
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453.
- Guan, Q.; Lin, G.; Gong, Y.; Wang, J.; Tan, W.; Bao, D.; Liu, Y.; You, Z.; Sun, X.; Wen, Z.; et al. Highly efficient self-healable and dual responsive hydrogel-based deformable triboelectric nanogenerators for wearable electronics. J. Mater. Chem. A 2019, 7, 13948–13955.
- Lei, Z.; Wu, P. A highly transparent and ultra-stretchable conductor with stable conductivity during large deformation. Nat. Commun. 2019, 10, 3429.
- Jin, X.; Shang, Y.; Zou, Y.; Xiao, M.; Huang, H.; Zhu, S.; Liu, N.; Li, J.; Wang, W.; Zhu, P. Injectable hypoxia-induced conductive hydrogel to promote diabetic wound healing. ACS Appl. Mater. Interfaces 2020, 12, 56681–56691.
- Chen, Y.; Wu, W.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Mechanical strong stretchable conductive multi-stimuli-responsive nanocomposite double network hydrogel as biosensor and actuator. J. Biomater. Sci. Polym. Ed. 2020, 31, 1770–1792.
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J. Mater. Chem. B 2021, 9, 2561–2583.
- Muthuswamy, S.; Viswanathan, A.; Yegappan, R.; Selvaprithiviraj, V.; Vasudevan, A.K.; Biswas, R.; Jayakumar, R. Antistaphylococcal and neutrophil chemotactic injectable κ-carrageenan hydrogel for infectious wound healing. ACS Appl. Bio Mater. 2019, 2, 378–387.
- Han, W.; Zhou, B.; Yang, K.; Xiong, X.; Luan, S.; Wang, Y.; Xu, Z.; Lei, P.; Luo, Z.; Gao, J.; et al. Biofilm-inspired adhesive and antibacterial hydrogel with tough tissue integration performance for sealing hemostasis and wound healing. Bioact. Mater. 2020, 5, 768–778.
- Annabi, N.; Rana, D.; Shirzaei Sani, E.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243.
- Tantiwatcharothai, S.; Prachayawarakorn, J. Property improvement of antibacterial wound dressing from basil seed (O. basilicum L.) mucilage- ZnO nanocomposite by borax cross-linking. Carbohydr. Polym. 2020, 227, 115360.
- Meng, L.; Fu, C.; Lu, Q. Advanced technology for functionalization of carbon nanotubes. Prog. Nat. Sci. 2009, 19, 801–810.
- Wu, Y.; Xia, M.; Fan, Q.; Zhu, M. Designable synthesis of nanocomposite hydrogels with excellent mechanical properties based on chemical cross-linked interactions. Chem. Commun. 2010, 46, 7790–7792.
- Yang, B.X.; Pramoda, K.P.; Xu, G.Q.; Goh, S.H. Mechanical reinforcement of polyethylene using polyethylene-grafted multiwalled carbon nanotubes. Adv. Funct. Mater. 2007, 17, 2062–2069.
- De France, K.J.; Chan, K.J.W.; Cranston, E.D.; Hoare, T. Enhanced mechanical properties in cellulose nanocrystal–poly(oligoethylene glycol methacrylate) injectable nanocomposite hydrogels through control of physical and chemical cross-linking. Biomacromolecules 2016, 17, 649–660.
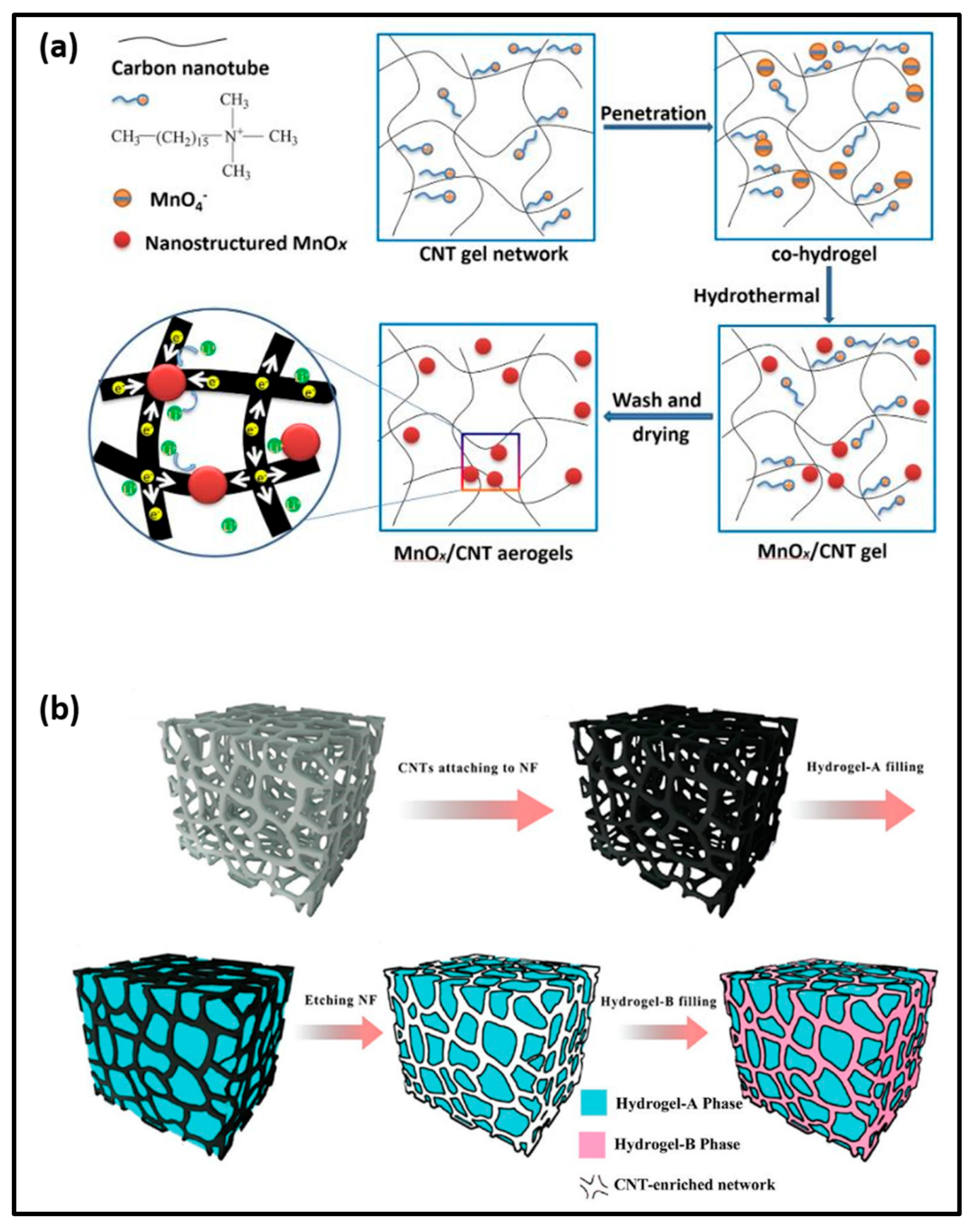
- Liu, Y.; Liu, M.; Zheng, P.; Ge, D.; Yang, L. Controllable hydrogel-thermal synthesis of Mn2O3/CNT aerogels: Shape evolution, growth mechanism and electrochemical properties. Mater. Des. 2019, 182, 108022.
- Ying, Z.; Wang, Q.; Xie, J.; Li, B.; Lin, X.; Hui, S. Novel electrically-conductive electro-responsive hydrogels for smart actuators with a carbon-nanotube-enriched three-dimensional conductive network and a physical-phase-type three-dimensional interpenetrating network. J. Mater. Chem. C 2020, 8, 4192–4205.
- Roy, S.; Das, T.; Ming, Y.; Chen, X.; Yue, C.Y.; Hu, X. Specific functionalization and polymer grafting on multi-walled carbon nanotubes to fabricate advanced nylon 12 composites. J. Mater. Chem. A 2014, 2, 3961–3970.
- Liu, S.; Gao, G.; Xiao, Y.; Fu, J. Tough and responsive oppositely charged nanocomposite hydrogels for use as bilayer actuators assembled through interfacial electrostatic attraction. J. Mater. Chem. B 2016, 4, 3239–3246.
- Ki, V.; Rotstein, C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184.
- Cardona, A.F.; Wilson, S.E. Skin and soft-tissue infections: A critical review and the role of telavancin in their treatment. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 61 (Suppl. 2), S69–S78.
- Andonova, M.; Urumova, V. Immune surveillance mechanisms of the skin against the stealth infection strategy of Pseudomonas aeruginosa—Review. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 433–448.
- Han, G.; Zhang, W. Improved biclique cryptanalysis of the lightweight block cipher piccolo. Secur. Commun. Netw. 2017, 2017, 7589306.
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526.
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789.