Lactoferrins are an iron-binding glycoprotein that have important protective roles in the mammalian body through their numerous functions, which include antimicrobial, antitumor, anti-inflammatory, immunomodulatory, and antioxidant activities. Among these, their antimicrobial activity has been the most studied, although the mechanism behind antimicrobial activities remains to be elucidated.
- lactoferrin
- lactoferricin
- peptides
- antimicrobial activity
- mechanisms of action
1. Introduction
Lactoferrins are iron-binding proteins that belong to the transferrin family. Since the first isolation of lactoferrins from both bovine [1] and human [2][3][2,3] milk in 1960, they have been the subject of intensive structural and functional studies, especially because of their numerous functions, properties, and applications in the food and pharmaceutical industries. Lactoferrins have also been identified in other mammalian species, as listed in Table 1 ; however, bovine and human lactoferrins have been the most studied to date.
| Order | Species | Source of Lactoferrin Isolation | Reference | |
|---|---|---|---|---|
| Primates | Human | Colostrum, milk, tears, nasal/bronchial secretions, saliva, bile/pancreatic secretions (i.e., gastric/intestinal fluids), urine, seminal/vaginal fluids, granules of neutrophils | [4][5][6][7] | [4,5,7,9] |
| Rhesus monkey | Milk | [8] | [10] | |
| Patas monkey, macaque, baboon, orangutan | Granules of neutrophils | [6] | [7] | |
| Carnivores | Dog, bear, domestic cat, tiger, jaguar, cougar, meerkat, otter, tayra, palm civet | Granules of neutrophils | [9][6] | [6,7] |
| Rodents | Rat, hamster, aguti | Granules of neutrophils | [6] | [7] |
| Mouse, guinea pig | Milk, granules of neutrophils | [4][6] | [4,7] | |
| Lagomorpha | Rabbit | Granules of neutrophils | [6][10] | [7,11] |
| Artiodactyla | Sheep, buffalo, alpaca, camel | Milk | [7][11][12] | [9,12,13] |
| Deer | Granules of neutrophils | [4][6] | [4,7] | |
| Cow, goat, pig | Milk, granules of neutrophils | [6] | [7] | |
| Perissodactyla | Horse | Milk, granules of neutrophils | [4][6] | [4,7] |
| Proboscidea | Elephant (Asian and African) | Milk | [7][13] | [9,14] |
| Didelphimorphia | Opossum | Granules of neutrophils | [6] | [7] |
| Cingulata | Armadillo | Granules of neutrophils | [6] | [7] |
Numerous functions have been attributed to lactoferrins, which have ranged from antimicrobial (i.e., antibacterial, antivirus, antifungal, and antiparasitic) to antitumor, anti-inflammatory, immunomodulatory, and antioxidant activities.
Antibacterial activity was the first of these to be ascribed to lactoferrins, which have been well studied [14][15][16][38,39,40], since they represent a great potential as a natural defense agent. Lactoferrin and lactoferrin-derived peptides not only have a broad specter of antibacterial activities against Gram-positive and Gram-negative bacteria and can be potentially used as natural antibiotic in human and veterinary medicine [14][38] but also have a broad spectrum of activities against enveloped and naked viruses [17][18][19][41,42,43], fungi, yeast [20][21][44,45], and parasites [21][45]. Furthermore, lactoferrin has also been proposed to be potent as a treatment drug in the current COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV 2) [22][23][24][25][26][46,47,48,49,50]. The antimicrobial activity of lactoferrin is related to its ability to chelate iron and thus to deprive microorganisms of these important nutrient, although lactoferrin also expresses antimicrobial activity in the iron-independent pathway by direct interaction with microorganisms.
As lactoferrins were shown to be in body fluids that usually interact with the surrounding environment and considering their broad activity against different microbes, it is initially believed that lactoferrins have an important role in the initiation of the immune system.
2. Antibacterial Activities of Lactoferrins and Lactoferrin-Derived Peptides
Another aspect of the lactoferrin antimicrobial activity might arise from the binding of bovine lactoferrin to porins. Porins are transmembrane proteins that form channels for nonspecific diffusion of hydrophilic solutes across the outer membrane of Gram-negative bacteria [27][66]. Binding of bovine lactoferrin to porins OmpF and OmpC has been demonstrated [28][29][82,83].
Arnold et al. (1982) showed that the preincubation of S. mutans with human lactoferrin reduced glucose uptake and inhibited the synthesis of lactic acid, which indicated that lactoferrins can also affect glucose metabolism [30][59]. Furthermore, synergistic actions of lactoferrins with lysozymes (the major enzymatic components in the granules of polymorphonuclear lymphocytes) [31][64], bacteriophages [32][86], and antibiotics against different bacteria have been demonstrated [33][85]. By releasing LPS from the Gram-negative bacteria outer membrane, lactoferrins increase the permeability of the outer bacterial membrane and the susceptibility of Gram-negative bacteria to lysozymes, which have an important role in mammal defense mechanisms [31][64].
For human lactoferricin, the number of amino acids and the structure originally reported by Bellamy et al. (1992a) indicated 47 amino acids (1–47 of human lactoferrin) and included a region homologous to bovine lactoferricin. The sequence of human lactoferricin was reported as two subfragments connected by disulfide bonds between cysteine residues: the linear residues 1 to 11 and the cyclic residues 12 to 47 ( Figure 12 C) [34][57]. However, Hunter et al. (2005) later reported human lactoferricin as a 49-amino-acid peptide. They also proposed different human lactoferricin structures that are cyclic, although with a continuous polypeptide chain ( Figure 12 D) [35][70].
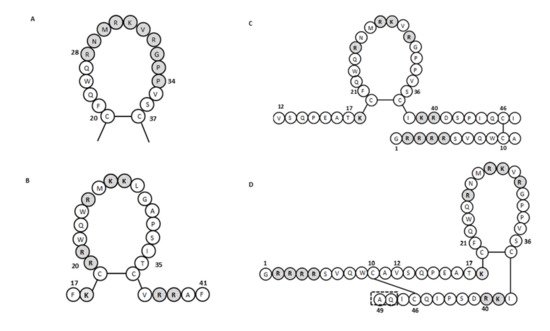
To determine the active regions of bovine and human lactoferricins, several synthetic peptides were made for each. All bovine lactoferricins inhibited bacterial growth; however, only to modified human lactoferricins showed activities against the bacteria tested, while non-modified peptides that corresponding to different regions of native human lactoferricin showed no antimicrobial activities [37][109]. In contrast, the small 11-amino-acid peptide (21–31 of human lactoferrin) that is homologous to the helical and loop regions of human lactoferrin and lactoferricin, respectively, inhibited growth of E. coli and bound LPS [38][104]. This thus indicated that this region has an important role in the antibacterial activity of human lactoferrin and lactoferricin.
3. Antifungal Activities of Lactoferrins and Lactoferrin-Derived Peptides
Antifungal activity for human lactoferrin was first reported by Kirkpatrick et al. (1971), where inhibition of growth was demonstrated against the yeast Candida albicans [39][199]. Human lactoferrin also inhibited the growth of Candida krusei , to a greater extent than seen for C. albicans . The inhibition of each of these was dose dependent [40][41][200,201], while iron saturation resulted in the loss of this antifungal activity of human lactoferrin [39][40][41][199,200,201]. Soon after the first isolation of bovine lactoferricin, its antifungal activity was extensively studied ( Supplementary Table S1 ), including for fungi that cause dermatophytosis. Using [14C]-labeled bovine lactoferricin, its direct binding was shown [42][202], along with its potent disruptive effects on C. albicans cell membranes [43][203]. Furthermore, for pathogenic fungi, bovine lactoferricin inhibited the uptake of [3H]-glucose in Trichophyton rubrum and caused substantial changes to the ultrastructure of Trichophyton mentagrophytes , which included dense aggregation of the cytoplasmic materials [44][204]. The anti- Candida activity of bovine and human lactoferricins can be affected by different pHs, temperatures, and ions (i.e., phosphate, bicarbonate, Ca 2+ , and Mg 2+ ) [41][42][201,202]. Indeed, bovine lactoferricin binding to C. albicans was reduced by the addition of the divalent cations Ca 2+ and Mg 2+ ,and was pH dependent, as also seen for its antimicrobial activity [42][202].
To further determine the antifungal activities of bovine lactoferricin, several lactoferricin-like peptides were tested by Ueta et al. (2001), using synthetic bovine lactoferricin and four of its derived peptides. Among these peptides, peptide 2 (amino acids 17–26) showed the greatest suppression of multiplication of Candida cells, while the other peptides showed only weak activities [45][205]. Munoz and Marcos (2006) tested two bovine lactoferricin-derived peptides, lactoferricin 17–31 and lactoferricin 20–25, against different bacteria and fungi that are causative agents for plant diseases [46][114], while van der Kraan et al. (2004) tested the anti- Candida activities of bovine lactoferricin fragment 17–30 [47][139]. These data showed that bovine lactoferricin (20–25) was less active than the more extended bovine lactoferricin (17–31), with the exception of Botrytis cinerea , where very similar activities were seen, and that filamentous fungi were more susceptible than bacteria or yeast [46][114]. Peptide 2 of Ueta et al. (2001) also did not bind iron, which indicated that its mechanism of anti- Candida activity was unrelated to depriving these yeast of this nutrient [45][205]. Three peptides obtained by tryptic digestion of bovine lactoferrin (i.e., 21LF, 38LF, and 45LF) showed lower antibacterial activities than the native protein; however, their antifungal activities were greater than that of lactoferrin [48][135]. Furthermore, the human lactoferricin 1–11 synthetic peptide inhibited biofilm formation by C. albicans mainly at the early stages, showing interference with the cell density of the biofilm and the metabolic activity [49][206].
Candida albicans has also shown high susceptibility to synthetic lactoferrampin 268–284 and other lactoferrampin-like peptides [47][50][51][139,140,207]. To define the antimicrobial region of bovine lactoferrampin, van der Kraan et al. (2005) synthesized a series of lactoferrampin peptides. They concluded that the positively charged amino acids of the C-terminal of lactoferrampin 265–284 were crucial for its candidacidal activity, while the N-terminal part was essential for activity because it facilitated helix formation [51][207]. Interestingly human lactoferrampin showed no inhibitory effects against C. albicans unless a lysine residue was added to the C-terminus of molecule or a negatively charged aspartic acid was mutated to asparagine [52][141].
4. Antiparasitic Activities of Lactoferrins and Lactoferrin-Derived Peptides
Lactoferrin and lactoferrin-derived peptides also exert antiparasitic activity against different protozoa and small parasites. Lactoferrin inhibited the in vitro growth of Babesia caballi and Babesia equi ; however, the inhibitory effect was greater for B. caballi and only occurred in the presence of apo form of lactoferrin [53][208]. Since many microorganisms, including parasites, require iron for growth and development, iron binding proteins lactoferrin can contribute to host defense against parasites by sequestrating this important nutrient from microorganisms [54][209]. Furthermore, human and bovine lactoferrin ant lactoferrin-derived peptides (including lactoferricin, lactoferampin, and Lf-chimera) also showed antiparasitic activity against Giardia lamblia [55][56][210,211] and Giardia intestinalis [57][212]. The most gardicidal effect was observed for bovine lactoferrin-derived peptides (50% lethal dose (LD 50s ) of 8 µg/mL) followed by human-derived peptides, bovine lactoferrin (LD 50s of 1.2 mg/mL), and human lactoferrin (LD 50s of 1.5 mg/mL), indicating that bovine lactoferrin is more potent that human lactoferrin [55][210]. Furthermore, it has been shown that lactoferrins and lactoferrin-derived peptides bind on the surface of G. lamblia [56][58][211,213]. The gardicidal effect of lactoferrin and lactoferrin-derived peptides was also observed at low concentrations, where they caused dilation of the endoplasmic reticulum (ER) membranes, expansion of the nuclear membrane, and plasma membrane protrusions [56][211], although high concentrations cause severe morphological changes or even induce programmed cell death [57][58][212,213]. Lactoferrin also exhibited antiparasitic activity against Cryptosporidium parvum sporozoites but had no significant effect on oocysts viability or parasite intracellular development [59][214].
Some protozoan parasites such as Trichomonas, Giardia, and Entamoeba require a high extracellular iron concentration for their growth [60][61][215,216] and have therefore adapted to acquire extracellular iron from other sources such as host iron-binding proteins such as transferrin and lactoferin. It is well known that Trypanosoma brucei can sequestrate iron from transferrin by binding through a surface receptor [62][217]. Iron uptake from transferrin and lactoferrin has also been demonstrated for Trichomonas vaginalis [61][216] , Trichomonas foetus [63][218], and Leishmania chagasi [64][219]; however, in the last case, other possible mechanism of iron sequestrating have been proposed [65][220]. Binding sites for lactoferrin has also been demonstrated in T. brucei [66][221] and Toxoplasma gondii [67][68][69][222,223,224]; however, in T. gondii, binding sites were specific for lactoferrin since the absence of transferrin binding was observed [69][224]. It is possible that protozoa binding sites for lactoferrin could have roles in iron acquisition; however, this is yet unclear since iron saturation had no impact on binding pattern [66][67][221,222]. Furthermore, studies by Tanaka et al. and Dzitoko et al. showed that lactoferrins did not prevent parasite penetration into host cell or had direct cytotoxic impact on T. gondii viability [70][71][72][225,226,227]; however, the inhibition of protozoa multiplication by lactoferrin was demonstrated [72][227]. Furthermore, when the lactoferrin-derived peptide lactoferricin was applied, a reduced viability, cyst formation in mouse brains, infectivity of sporozoites, and decreased penetration activity by T. gondii was observed [70][73][74][225,228,229]. Reduced infectivity of sporozoites was also observed in a case of Eimeria stiedai [74][229].
