hHeart failure (HF) is characterized by a progressive course of disease accompanied by recurrent exacerbations leading to high hospitalization and rehospitalization rates, which account for a substantial part of the disease load. Among the elderly, acute decompensated heart failure (ADHF) is the leading cause of hospitalization. The 30-day rehospitalization rate following the first admission to the hospital for HF exacerbation is 22–29.4%, which is the most common amongst all other etiologies.
- heart failure
- remote patient monitoring
- telemedicine
- preventive medicine
- rehospitalization
1. Introduction
Heart failure (HF) is a common clinical syndrome with detrimental effects at the individual patient and society levels [1][2]. Overall, 2.2% of US adults or nearly 6.2 million individuals suffer from HF, imposing a significant yearly financial burden estimated at 30.7 billion US dollars in 2012, and projected to more than double by 2030 [3]. The prevalence of HF is expected to continuously grow due to medical and societal developments [3]. First, HF is rising as the population ages, reaching more than 12% in older adults above the age of 80 [4]. Second, the improvement in treatments of HF has led to lower mortality rates, leaving more patients in need of chronic care [5]. Third, the rise in obesity and metabolic syndrome incidence is another contributor to the increased prevalence of HF cases [3][6][7][8]. Recently, advances in HF drug and device therapies have brought about impactful achievements to the field, yet a quarter of patients will endure considerable symptoms, hospitalizations, and mortality, despite optimal medical treatment. Consequently, additional approaches to further improve the management of HF are essential [6][9][10].
HF is characterized by a progressive course of disease accompanied by recurrent exacerbations leading to high hospitalization and rehospitalization rates, which account for a substantial part of the disease load [11][12][13][14][15]. Among the elderly, acute decompensated heart failure (ADHF) is the leading cause of hospitalization [3]. The 30-day rehospitalization rate following the first admission to the hospital for HF exacerbation is 22–29.4%, which is the most common amongst all other etiologies [16][17][18][19]. Likewise, ADHF is a leading cause (8.6%) of rehospitalization following hospitalization for other etiologies [16]. Moreover, ADHF admission is associated with poor quality of life, and approximately one-third of patients die within a year after an index admission [20][21][22]. Given the high prevalence and financial incentive, reducing HF hospitalization and readmission rates has become a foremost priority in the health systems [23][24].
Understanding the time course of progression to ADHF, it has been hypothesized that interventions to achieve an euvolemic status prior to overt clinical manifestation may prevent HF exacerbation events [25][26]. Several approaches to monitor HF patients have been tested, aiming to detect early warning signs of HF exacerbation. Daily monitoring of weight gain has not been successful in reducing rehospitalization or death rates as compared to control cases in weight monitoring in patients with severe heart failure (WISH) trial involving 344 in-hospital patients with ADHF [27]. In other large-scale studies, weight monitoring did not predict HF rehospitalizations [28].
In the Trans-European Network-Home-Care Management System trial (TEN-HMS), 426 patients with recent HF-related hospitalizations were randomized into three monitoring options: home telemonitoring (HTM), monthly nurse telephone support (NTS), or usual care (UC). Home telemonitoring (consisting of twice-daily measurements of body weight, blood pressure, and heart rate and rhythm by automated devices) was reviewed by a care manager at a linked medical reference center to facilitate prompt intervention when needed. Patients randomized to HTM or NTS had significantly lower mortality rates at 240 days post hospitalization compared with UC (29%, 27%, and 45%, respectively, p = 0.032). There was no difference in mortality or HF admission between HTM and NTS, but the length of stay in the hospital was six days shorter for the HTM arm [29]. Supporting results were also obtained from the Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial, and in trials focusing on the Heart-Mobile program, demonstrating improved hospitalization indices and reduced all-cause mortality in similarly monitored participants [30][31]. In contrast, the Better Effectiveness After Transition–Heart Failure (BEAT-HF) randomized trial involving 1437 patients who were discharged home with comparable monitoring methods after HF hospitalization has failed to demonstrate a favorable clinical outcome at 180 days of follow-up [32].
2. Non-Invasive Heart F ailure (HF) Monitoring
The fluid content range in the lungs is 20 to 35% in normal conditions, above which pulmonary edema may occur [23]. Residual pulmonary congestion at the time of discharge after hospitalization for ADHF is a strong predictor of rehospitalization [33]. Pulmonary congestion develops prior to clinical evidence of ADHF ( Figure 1 ), thus making it an attractive target for monitoring by several non-invasive technologies.
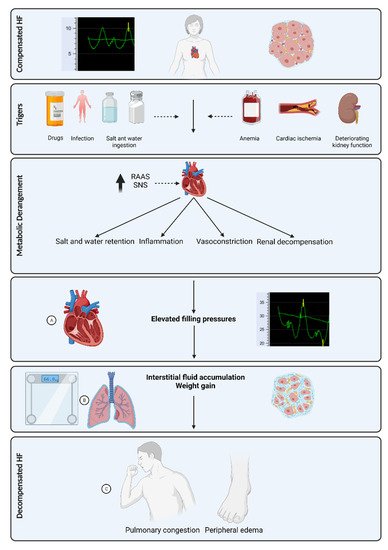
Impedance techniques in lung water measurements are based on the principle that air and water have different resistance. When water fills the lungs, conductance increases and impedance decreases [34][35][36][37]. Lung impedance (LI) monitoring using Edema-Guard monitor (CardioSet Company Ltd., Tel Aviv, Israel) once a month at ambulatory clinic visits demonstrated decreased HF hospitalization and mortality rate in a randomized controlled trial involving 256 HF patients [38]. Moreover, as measured by LI during HF hospitalization, the improvement in pulmonary fluid volume was predictive of lower readmission rate and demonstrated a better correlation than other clinical measures, such as N-terminal pro-brain natriuretic peptide (NT pro-BNP) or weight [39][40][41].
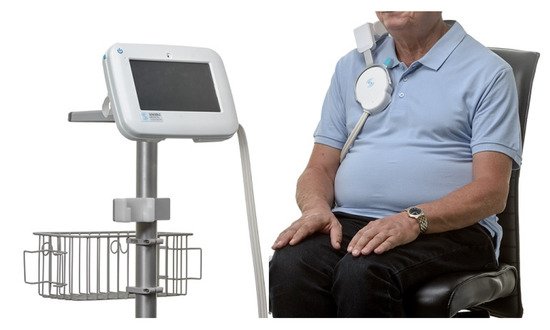
The Remote Dielectric Sensing (ReDS) vest (Sensible Medical Innovations Ltd., Netanya, Israel), measures the lung fluid content using a focused electromagnetic beam similar to radar technology ( Figure 2 ). When compared to right heart catheterization in HF patients, readings of >34% fluid content were highly correlated to pulmonary capillary wedge pressure > 18 mmHg (area under the curve (AUC) of 0.848, a sensitivity of 90.7%, and a specificity of 77.1%) [42]. Furthermore, results from 24 patients hospitalized for ADHF showed a correlation between the reduction in ReDS values and reduced pulmonary congestions and net fluid balance [43]. In another study, including 47 patients hospitalized for HF, hospitalization rates before and after the index hospitalization were compared without and with the use of ReDS vest, showing a significant reduction in HF readmissions with ReDS vest technology [44]. Additionally, preliminary results from a multicenter trial randomizing 268 patients to monitoring-guided or standard medical therapy following ADHF hospitalization demonstrated a 48% (95% CI: 31–87%, p = 0.01) reduction in 9 months rehospitalization in the ambulatory ReDS monitoring group [45].
3. Invasive HF Monitoring
Notably, previous studies have demonstrated that an increase in filling pressure may precede HF decompensation by three weeks or even more [46]. These findings have accelerated the search for effective and reliable devices that can consistently transmit intracardiac pressure readings to identify filling pressure rise early before clinical deterioration occurs. Two decades ago, an implantable hemodynamic monitoring device implanted in the right ventricle as a pacemaker was introduced. The device recorded various parameters, such as heart rate, right ventricle systolic and diastolic blood pressure, and estimated diastolic pulmonary artery (PA) pressure [47][48]. This device opened the door for an era of advanced invasive monitoring in the setting of HF.
Given these promising results, CardioMEMS was approved by the FDA in 2014 [49], and real-world data from several retrospective cohort studies demonstrated similar results [50][51]. In the European Society of Cardiology (ESC) guidelines from 2021, monitoring of PA pressure using a wireless implantable hemodynamic monitoring system (CardioMEMS) received a class IIb recommendation for symptomatic patients with HF and a previous HF hospitalization [52].
Whereas CardioMEMS and other PA sensors measure right-sided pressures, left-side filling pressure measurements may provide additional important information regarding the patient’s tendency for pulmonary congestion. In animal models, an increase in left atrial (LA) pressure significantly correlated with pulmonary congestion, and reversal of pressure elevation resulted in normalization of lung permeability. In addition, many factors contribute to a mismatch between PA and LA pressure, including elevated pulmonary vascular resistance, advanced HF, acute HF, and pulmonary hypertension [53]. These data suggest that PA pressure measurements may be inaccurate in estimating LV filling pressure.
A novel LA pressure sensor that is currently being investigated is the V-LAP system (Vectorious Medical Technologies, Tel Aviv, Israel). The V-LAP system is a wireless sensor that uses a MEMS pressure transducer and is implanted in the interatrial septum under angiographic and echocardiographic guidance ( Figure 43 ). In preclinical phases, the V-LAP system was implanted in 10 ovines, and its measurements were compared with postcapillary wedge pressure (PCWP) obtained by right heart catheterization at 1, 2, and 3–6 months after implantation. The mean difference was 0.19 ± 2.51 mmHg, and a strong correlation between V-LAP and PCWP measurements was observed, with r = 0.97 [54]. Short reports regarding patients implanted with the V-LAP system have similarly indicated significant correlations between PCWP measurements using right heart catheterization and LA pressure measurements, and when appropriate, clinical responses to an increased dose of diuretics in patients with high pressures measured by V-LAP has been observed [55][56]. An ongoing single-arm, open-label pilot clinical trial, the VECTOR-HF (V-LAP Left Atrium Monitoring systEm for Patients With Chronic sysTOlic & Diastolic Congestive heaRt Failure), is designed to assess the safety of the V-LAP system in patients with HF.

4. Conclusions
Early detection and intervention in HF patients to prevent clinical HF decompensation and subsequent hospitalization may provide significant health and financial advantages. In contrast to previous monitoring methods, novel technologies have been developed to target the initial aspects of the pathophysiological cascade of HF decompensation. Invasive and non-invasive methods have remarkably advanced cardiovascular medicine, taking advantage of recent developments in MEMS, big data, artificial intelligence, and wearable sensors. There is a growing body of evidence supporting a potential clinical benefit from monitoring devices for the management of HF, mainly with PA pressure monitoring. Table 1 summarizes the key clinical trials published recently on different ambulatory heart failure monitoring technologies and their main findings. In the future, alongside further technological advances, appropriate integration of patient monitoring into the clinical workflow will help make the most of these exciting devices.
| Year | Reference | Patient Characteristics | Monitoring Method | Follow Up | Primary Endpoint | Secondary Endpoint |
|---|---|---|---|---|---|---|
| 2012 | WISH [27] | 344 patients hospitalized for ADHF and NFYHA III-IV, LVEF < 50% | Daily weighing using internet connected scale. | 12 months | No difference in cardiac re-hospitalizations (HR 0.90, CI 0.65–1.26, p = 0.54) | No difference in all cause hospitalization, death, or composite of both. |
| 2005 | TEN-HMS [29] | 426 patients with in 6 weeks of ADHF admission and LVEF <40% and on diuretics | Home telemonitoring (automatic BP, electronic scale, ECG), monthly nurse phone call or usual care | 240 days | Days lost for death or hospitalization did not differ (12.7%, 15.9%, 19.5% respectively) | Mortality was higher in usual care group (45 vs. 27% in nurse phone call and 29% in telemonitoring groups) |
| 2016 | BEAT-HF [32] | 1437 patients hospitalized for ADHF | electronic telemonitoring (BP, heart rate, weight, symptoms) + monthly tele-coaching or usual care | 180 days | Similar all cause hospitalization at 180 days- 50.8% vs. 49.2% respectively (HR-1.03; 95% CI, 0.88–1.20; p = 0.74) | no significant differences in 30-day readmission or 180-day mortality. |
| 2016 | IMPEDANCE-HF [38] |
256 patients with ADHF admission in the last year, LVEF < 35%, NYHA II-IV | Monthly lung impedance vs. usual care | 48 ± 32 months | 211 vs. 386 ADHF hospitalizations (p < 0.001) among monitored vs. control | 42 vs. 59 deaths respectively (HR 0.52, 95% CI 0.35–0.78, p = 0.002) |
| 2019 | SMILE [45] (Preliminary results) |
268 patients with current ADHF hospitalization | Remote dielectric sensing vs. usual care | 6.1 ± 3.4 months | 21 vs. 43 readmissions (HR 0.52, 95% CI- 0.31–0.87, p = 0.01) | No mortality benefits. Lower days lost for ADHF (1.37 vs. 2.62, p = 0.006) |
| 2014 | IN-TIME [57] | 664 patients, LVEF < 35%, NYHA II-III, OMT. | CIED based daily monitoring (HR, activity, arrythmia, HR, HR variability, HR at rest, ventricular ectopy) vs. usual care | 12 months | Composite of all-cause death, overnight hospital admission for heart failure, change in NYHA class patient global self-assessment was better in monitored group (18.9% vs. 27.2%, OR 0.63, 95% CI 0.43–0.90, p = 0·013) | Mortality of 10 vs. 27 patients respectively. |
| 2011 | DOT-HF [58] | 335 patients with ADHF admission in the last year, LVEF < 35%, NYHA II-IV | CIED based thoracic impedance monitoring vs. usual care | 14.9 ± 5 months | all-cause mortality and HF hospitalizations was similar (29% vs. 20% (p = 0.063, HR 0.52; 95% CI- 0.97–2.37) | HF hospitalization (HR0 1.79; 95% CI- 1.08–2.95; p = 0.022) and outpatient visits (250 vs. 84, p < 0.0001) were higher in the monitored group |
| 2011 | COMPASS-HF [48] | 274 HF patients, on OMT, NYHA III-IV and ADHF hospitalization in previous 6 months | Implantable RV and ePAD pressure monitor | 6 months | Nonsignificant 21% reduction in HF hospitalizations (p = 0.33) | time to first HF-related hospitalizations was 35% lower (HR-0.64, 95% CI-0.42–0.96, p = 0.03) |
| 2016 | CHAMPION [59] | 550 HF patients with previous ADHF hospitalization and NYHA III | Implantable PA pressure monitor | 18 months (complete follow up) | ADHF admissions were 33% lower (HR- 0.67, 95% CI 0.55–0.80, p < 0.0001) |
References
- Rossignol, P.; Hernandez, A.F.; Solomon, S.D.; Zannad, F. Heart failure drug treatment. Lancet 2019, 393, 1034–1044.
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995.
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619.
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, e2–e220.
- Barker, W.H.; Mullooly, J.P.; Getchell, W. Changing incidence and survival for heart failure in a well-defined older population, 1970-1974 and 1990-1994. Circulation 2006, 113, 799–805.
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603.
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528.
- Cook, C.; Cole, G.; Asaria, P.; Jabbour, R.; Francis, D.P. The annual global economic burden of heart failure. Int. J. Cardiol. 2014, 171, 368–376.
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486.
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; Michl, K.; et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009, 119, e391–e479.
- Abid, L.; Charfeddine, S.; Kammoun, I.; Halima, M.B.; Slima, H.B.; Drissa, M.; Mzoughi, K.; Mbarek, D.; Riahi, L.; Antit, S.; et al. Epidemiology of heart failure and long-term follow-up outcomes in a north-African population: Results from the NAtional TUnisian REgistry of Heart Failure (NATURE-HF). PLoS ONE 2021, 16, e0251658.
- Dunlay, S.M.; Shah, N.D.; Shi, Q.; Morlan, B.; VanHouten, H.; Long, K.H.; Roger, V.L. Lifetime costs of medical care after heart failure diagnosis. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 68–75.
- Bello, N.A.; Claggett, B.; Desai, A.S.; McMurray, J.J.V.; Granger, C.B.; Yusuf, S.; Swedberg, K.; Pfeffer, M.A.; Solomon, S.D. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ. Heart Fail. 2014, 7, 590–595.
- Solomon, S.D.; Dobson, J.; Pocock, S.; Skali, H.; McMurray, J.J.V.; Granger, C.B.; Yusuf, S.; Swedberg, K.; Young, J.B.; Michelson, E.L.; et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007, 116, 1482–1487.
- Bhatia, R.S.; Tu, J.V.; Lee, D.S.; Austin, P.C.; Fang, J.; Haouzi, A.; Gong, Y.; Liu, P.P. Outcome of heart failure with preserved ejection fraction in a population-based study. N. Engl. J. Med. 2006, 355, 260–269.
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N. Engl. J. Med. 2009, 360, 1418–1428.
- Strom, J.B.; Kramer, D.B.; Wang, Y.; Shen, C.; Wasfy, J.H.; Landon, B.E.; Wilker, E.H.; Yeh, R.W. Short-term rehospitalization across the spectrum of age and insurance types in the United States. PLoS ONE 2017, 12, e0180767.
- Joynt, K.E.; Jha, A.K. Who has higher readmission rates for heart failure, and why? Implications for efforts to improve care using financial incentives. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 53–59.
- Dharmarajan, K.; Hsieh, A.F.; Lin, Z.; Bueno, H.; Ross, J.; Horwitz, L.; Barreto-Filho, J.A.; Kim, N.; Bernheim, S.M.; Suter, L.G.; et al. Diagnoses and Timing of 30-Day Readmissions After Hospitalization for Heart Failure, Acute Myocardial Infarc-tion, or Pneumonia. JAMA 2013, 309, 355–363.
- Loehr, L.R.; Rosamond, W.D.; Chang, P.P.; Folsom, A.R.; Chambless, L.E. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am. J. Cardiol. 2008, 101, 1016–1022.
- Rich, M.W.; Beckham, V.; Wittenberg, C.; Leven, C.L.; Freedland, K.E.; Carney, R.M. A Multidisciplinary Intervention to Prevent the Readmission of Elderly Patients with Congestive Heart Failure. N. Engl. J. Med. 1995, 333, 1190–1195.
- Kitzman, D.W.; Whellan, D.J.; Duncan, P.; Pastva, A.M.; Mentz, R.J.; Reeves, G.R.; Nelson, M.B.; Chen, H.; Upadhya, B.; Reed, S.D.; et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N. Engl. J. Med. 2021, 385, 203–216.
- Bekfani, T.; Fudim, M.; Cleland, J.G.F.; Jorbenadze, A.; von Haehling, S.; Lorber, A.; Rothman, A.M.K.; Stein, K.; Abraham, W.T.; Sievert, H.; et al. A current and future outlook on upcoming technologies in remote monitoring of patients with heart failure. Eur. J. Heart Fail. 2021, 23, 175–185.
- Cheng, R.; Cox, M.; Neely, M.L.; Heidenreich, P.A.; Bhatt, D.L.; Eapen, Z.J.; Hernandez, A.F.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare popultion. Am. Hear. J. 2014, 168, 721–730.
- Radhoe, S.P.; Veenis, J.F.; Brugts, J.J. Invasive Devices and Sensors for Remote Care of Heart Failure Patients. Sensors 2021, 21, 2014.
- Kapelios, C.J.; Malliaras, K.; Kaldara, E.; Vakrou, S.; Nanas, J.N. Loop diuretics for chronic heart failure: A foe in disguise of a friend? Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 54–63.
- Lyngå, P.; Persson, H.; Martinell, A.H.; Hägglund, E.; Hagerman, I.; Langius-Eklöf, A.; Rosenqvist, M. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur. J. Hear. Fail. 2012, 14, 438–444.
- Zhang, J.; Goode, K.M.; Cuddihy, P.E.; Cleland, J.G.F.; TEN-HMS Investigators. Predicting hospitalization due to worsening heart failure using daily weight measurement: Analysis of the Trans-European Network-Home-Care Management System (TEN-HMS) study. Eur. J. Heart Fail. 2009, 11, 420–427.
- Cleland, J.G.F.; Louis, A.A.; Rigby, A.S.; Janssens, U.; Balk, A.H.M.M.; TEN-HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: The Trans-European Network-Home-Care Management System (TEN-HMS) study. J. Am. Coll. Cardiol. 2005, 45, 1654–1664.
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057.
- Poelzl, G.; Egelseer-Bruendl, T.; Pfeifer, B.; Modre-Osprian, R.; Welte, S.; Fetz, B.; Krestan, S.; Haselwanter, B.; Zaruba, M.M.; Doerler, J.; et al. Feasibility and effectiveness of a multidimensional post-discharge disease management programme for heart failure patients in clinical practice: The HerzMobil Tirol programme. Clin. Res. Cardiol. 2021.
- Ong, M.K.; Romano, P.S.; Edgington, S.; Aronow, H.U.; Auerbach, A.D.; Black, J.T.; de Marco, T.; Escarce, J.J.; Evangelista, L.S.; Hanna, B.; et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients With Heart Failure: The Better Effectiveness After Transition—Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 310–318.
- Coiro, S.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Alunni, G.; Murrone, A.; Tritto, I.; Zannad, F.; Girerd, N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur. J. Heart Fail. 2015, 17, 1172–1181.
- Peacock, W.I.; Albert, N.M.; Kies, P.; White, R.D.; Emerman, C.L. Bioimpedance monitoring: Better than chest x-ray for predicting abnormal pulmonary fluid? Congest. Heart Fail. 2000, 6, 86–89.
- Ramos, M.U.; LaBree, J.W.; Remole, W.; Kubicek, W.G. Transthoracic electric impedance. A clinical guide of pulmonary fluid accumulation in congestive heart failure. Minn. Med. 1975, 58, 671–676.
- Ito, H.; Yamakoshi, K.I.; Togawa, T. Transthoracic admittance plethysmograph for measuring cardiac output. J. Appl. Physiol. 1976, 40, 451–454.
- Patterson, R.P.; Kubicek, W.G.; Witsoe, D.A.; From, A.H. Studies on the effect of controlled volume change on the thoracic electrical impedance. Med. Biol. Eng. Comput. 1978, 16, 531–536.
- Shochat, M.K.; Shotan, A.; Blondheim, D.S.; Kazatsker, M.; Dahan, I.; Asif, A.; Rozenman, Y.; Kleiner, I.; Weinstein, J.M.; Frimerman, A.; et al. Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients: A Randomized Controlled Trial (IMPEDANCE-HF Trial). J. Card. Fail. 2016, 22, 713–722.
- Shochat, M.K.; Fudim, M.; Shotan, A.; Blondheim, D.S.; Kazatsker, M.; Dahan, I.; Asif, A.; Rozenman, Y.; Kleiner, I.; Weinstein, J.M.; et al. Prediction of readmissions and mortality in patients with heart failure: Lessons from the IMPEDANCE-HF extended trial. ESC Heart Fail. 2018, 5, 788–799.
- Shochat, M.; Charach, G.; Meyler, S.; Kazatzker, M.; Mosseri, M.; Frimerman, A.; Rabinovich, P.; Shotan, A.; Meisel, S. In-ternal thoracic impedance monitoring: A novel method for the preclinical detection of acute heart failure. Cardiovasc. Revasc. Med. 2006, 7, 41–45.
- Shochat, M.; Shotan, A.; Blondheim, D.S.; Kazatsker, M.; Dahan, I.; Asif, A.; Shochat, I.; Frimerman, A.; Rozenman, Y.; Meisel, S.R. Derivation of baseline lung impedance in chronic heart failure patients: Use for monitoring pulmonary congestion and predicting admissions for decompensation. J. Clin. Monit. Comput. 2015, 29, 341–349.
- Uriel, N.; Sayer, G.; Imamura, T.; Rodgers, D.; Kim, G.; Raikhelkar, J.; Sarswat, N.; Kalantari, S.; Chung, B.; Nguyen, A.; et al. Relationship Between Noninvasive Assessment of Lung Fluid Volume and Invasively Measured Cardiac Hemodynamics. J. Am. Heart Assoc. 2018, 7, e009175.
- Amir, O.; Rappaport, D.; Zafrir, B.; Abraham, W.T. A novel approach to monitoring pulmonary congestion in heart failure: Initial animal and clinical experiences using remote dielectric sensing technology. Congest. Heart Fail. 2013, 19, 149–155.
- Abraham, W.T.; Amir, O.; Weinstein, J.M.; Abbo, A.; Tuvia, B.G. Remote Dielectric Sensing (ReDS™)—Guided Patient Management of Ambulatory Heart Failure Patients Reduces Rehospitalization Rates. J. Card. Fail. 2015, 21 (Suppl. S77).
- Abraham, W.T.; Anker, S.; Burkhoff, D.; Cleland, J.; Gorodeski, E.; Jaarsma, T.; Small, R.; Lindenfeld, J.; Miller, A.; Ogenstad, S.; et al. Primary Results of the Sensible Medical Innovations Lung Fluid Status Monitor Allows Reducing Readmission Rate of Heart Failure Patients (smile) Trial. J. Card. Fail. 2019, 25, 938.
- Adamson, P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: New insights from continuous monitoring devices. Curr. Heart Fail. Rep. 2009, 6, 287–292.
- Magalski, A.; Adamson, P.; Gadler, F.; Böehm, M.; Steinhaus, D.; Reynolds, D.; Vlach, K.; Linde, C.; Cremers, B.; Sparks, B.; et al. Continuous ambulatory right heart pressure measurements with an implantable hemodynamic monitor: A multicenter, 12-month follow-up study of patients with chronic heart failure. J. Card. Fail. 2002, 8, 63–70.
- Bourge, R.C.; Abraham, W.T.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M., Jr.; Magalski, A.; Zile, M.R.; Smith, A.L.; Smart, F.W.; O’Shaughnessy, M.A.; et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: The COMPASS-HF study. J. Am. Coll. Cardiol. 2008, 51, 1073–1079.
- SFDA. PMA Number P100045. 2014. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100045 (accessed on 10 October 2021).
- Desai, A.S.; Bhimaraj, A.; Bharmi, R.; Jermyn, R.; Bhatt, K.; Shavelle, D.; Redfield, M.M.; Hull, R.; Pelzel, J.; Davis, K.; et al. Ambulatory Hemodynamic Monitoring Reduces Heart Failure Hospitalizations in "Real-World" Clinical Practice. J. Am. Coll. Cardiol. 2017, 69, 2357–2365.
- Abraham, J.; Bharmi, R.; Jonsson, O.; Oliveira, G.H.; Artis, A.; Valika, A.; Capodilupo, R.; Adamson, P.B.; Roberts, G.; Dalal, N.; et al. Association of Ambulatory Hemodynamic Monitoring of Heart Failure With Clinical Outcomes in a Concurrent Matched Cohort Analysis. JAMA Cardiol. 2019, 4, 556–563.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chion-cel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Abraham, W.T.; Perl, L. Implantable Hemodynamic Monitoring for Heart Failure Patients. J. Am. Coll. Cardiol. 2017, 70, 389–398.
- Perl, L.; Soifer, E.; Bartunek, J.; Erdheim, D.; Köhler, F.; Abraham, W.T.; Meerkin, D. A Novel Wireless Left Atrial Pressure Monitoring System for Patients with Heart Failure, First Ex-Vivo and Animal Experience. J. Cardiovasc. Transl. Res. 2019, 12, 290–298.
- D’Amario, D.; Restivo, A.; Canonico, F.; Rodolico, D.; Mattia, G.; Francesco, B.; Vergallo, R.; Trani, C.; Aspromonte, N.; Crea, F. Experience of remote cardiac care during the COVID-19 pandemic: The V-LAP™ device in advanced heart failure. Eur. J. Heart Fail. 2020, 22, 1050–1052.
- Feickert, S.; D’Ancona, G.; Murero, M.; Ince, H. Intra-cardiac microcomputer allows for innovative telemedicine in chronic heartfailure during coronavirus disease-2019 pandemic: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–6.
- Hindricks, G.; Taborsky, M.; Glikson, M.; Heinrich, U.; Schumacher, B.; Katz, A.; Brachmann, J.; Lewalter, T.; Goette, A.; Block, M.; et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): A randomised controlled trial. Lancet 2014, 384, 583–590.
- van Veldhuisen, D.J.; Braunschweig, F.; Conraads, V.; Ford, I.; Cowie, M.R.; Jondeau, G.; Kautzner, J.; Aguilera, R.M.; Lunati, M.; Yu, C.M.; et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011, 124, 1719–1726.
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B.; CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet 2016, 387, 453–461.
