Protein glycosylation is a highly conserved post-translational modification among organisms. It plays fundamental roles in many biological processes, ranging from protein trafficking and cell adhesion to host–pathogen interactions. According to the amino acid side chain atoms to which glycans are linked, protein glycosylation can be divided into two major categories: N-glycosylation and O-glycosylation. However, there are other types of modifications such as the addition of GPI to the C-terminal end of the protein. Besides the importance of glycoproteins in biological functions, they are a major component of the fungal cell wall and plasma membrane and contribute to pathogenicity, virulence, and recognition by the host immunity. Given that this structure is absent in host mammalian cells, it stands as an attractive target for developing selective compounds for the treatment of fungal infections.
1. Introduction
Protein glycosylation is a highly conserved post-translational modification found in both prokaryotic and eukaryotic cells characterized by the addition of oligosaccharides and glycolipids to the peptide backbone. Glycans influence biological processes, such as protein folding, targeted transport, cellular localization, and mediation of host–pathogen interactions, among others
[1]. Glycosylation is carried out by different mechanisms that occur in different cell compartments. According to the amide or hydroxyl groups of amino acids to which glycans are attached, glycoproteins are divided into
N-linked glycoproteins or
O-linked glycoproteins, although other types of glycoproteins have been described that are not part of this work. In the former, glycans are linked to asparagine residues, contained within a consensus sequon, Asn-X-Ser/Thr (where X is any amino acid except for proline)
[2]; meanwhile, in the latter, glycans are attached to a subset of serines, threonines, or hydroxylysines
[3][4][5][3,4,5].
Another post-translational modification is the anchoring of cell membrane and cell wall proteins to glycosylphosphatidylinositol (GPI) covalently attached to the
C-terminus. GPI biosynthesis is a multistep conserved pathway in eukaryotes that culminates in the generation of GPI glycolipids that anchor many proteins to the cell surface. GPI anchor synthesis appears to be essential in lower eukaryotes, affecting growth and viability
[6]. Additionally, there is evidence that GPI-anchored proteins are important for the pathogenicity and virulence of many pathogens, as discussed in the following sections.
As mentioned, glycoproteins are involved in a wide range of biological processes which ensure cell stability
[7]. For instance, they are a major constituent of the fungal cell wall and plasma membrane, and the biosynthetic pathways of these glycans have been thoroughly dissected in organisms such as
Candida albicans and
Saccharomyces cerevisiae. Rather than being a rigid and impenetrable shield, the fungal wall is plastic, permeable, and a molecular scaffold to display molecules to respond to changes in the environment or stresses. In addition, it is paramount for viability, morphogenesis, and pathogenesis
[8]. This wall is composed of structural polysaccharides (glucans, chitin, and chitosan) and GPI-,
N-linked, and
O-linked glycoproteins
[8]. Moreover, in fungal infections, the cell wall is one of the first points of contact with the host, being essential for fungal pathogenicity and virulence as well as the host’s immune response
[9]. The cell wall plays a major role during fungal invasion. It is capable of inducing immune responses due to the components that are not found in the host cells, through the recognition of cell surface fungal-specific molecules, named pathogen-associated molecular patterns, by pattern recognition receptors
[10]. Some of the wall antigens are also directly involved in fungal adhesion and colonization of the host tissues
[11].
2. The Candida albicans Protein Glycosylation Pathways
C. albicans is one of the most common human fungal pathogens that cause benign superficial infections of the mucosa and life-threatening systemic infections
[12]. The deep-seated infection is associated with high mortality rates, and treatment is becoming challenging because of the increased number of isolates showing drug resistance to the conventional therapeutic options
[12]. Therefore, the
C. albicans cell wall biosynthetic routes, including protein glycosylation, and the interaction of the cell wall components with the host immunity have attracted attention in recent decades, with the idea to find targets for the development of new antifungal drugs or immunomodulatory therapies. Since this organism has been regarded as a biological model of a fungal pathogen, its protein glycosylation has been thoroughly studied, and here, we will take these pathways as an example to describe
N-linked and
O-linked glycosylation, and GPI anchor synthesis.
2.1. The N-Linked Glycosylation Pathway
The first steps of
N-linked protein glycosylation are conserved among eukaryotes, such as fungi, plants, protozoa, and mammalian cells, although some peculiarities have been reported in particular species
[13][14][15][16][13,14,15,16]. To obtain the final structure displayed on glycoproteins,
N-linked glycans must undergo a series of modifications carried out by glycosidases and glycosyltransferases in the endoplasmic reticulum (ER) and the Golgi complex. This processing can be divided into two sequential stages: (a) the assembly of an oligosaccharide on an isoprenoid lipid in the rough ER by a set of specific glycosyltransferases, and its subsequent translocation to the nascent protein, and (b) further modification of an
N-linked glycan by ER glycosidases and Golgi glycosyltransferases
[13][17][18][13,17,18]. The enzymes involved in
N-linked glycan synthesis are highlighted in
Figure 1.
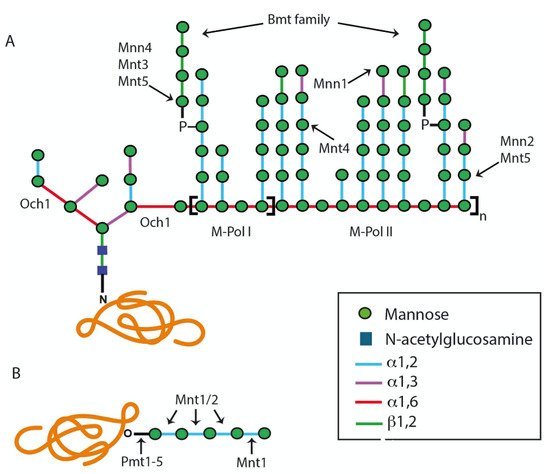
Figure 1. Schematic diagram representing the Candida albicans N-linked and O-linked glycans. (A) N-linked glycan. The outer chain elaboration begins with the addition of an α-1,6-mannose residue to the N-linked glycan core by the α-1,6-mannosyltransferase Och1. Then, an α-1,6-mannose backbone is synthesized by M-Pol I and M-Pol II complexes, and this is branched with α-1,2-mannooligomers that can be decorated with phosphomannan, β-1,2-mannose, or α-1,3-mannose units. (B) O-linked glycan. The addition of the first mannose residue is initiated in the endoplasmic reticulum by protein mannosyltransferases encoded by the PMT gene family. The glycoprotein is then transported to the Golgi complex for the addition of further mannose residues by mannosyltransferases Mnt1 and Mnt2.
2.1.1. Assembly of Lipid-Linked Oligosaccharide
The lipid moiety of the intermediates involved in protein glycosylation reactions is a polymer of isoprene units (CH3–C(CH3) = CH–CH2–), dolichol (Dol)
[2][19][20][2,19,20]. In
C. albicans, as in other eukaryotic cells, the synthesis of Dol-PP-GlcNAc
2Man
5 is achieved in the ER cytoplasmatic face by the addition of N-acetyl-D-glucosamine (GlcNAc) and mannose (Man), using UDP-GlcNAc and GDP-Man as donor substrates
[21]. Each of the sugar residues is added to the lipid-linked precursor by a specific glycosyltransferase; Alg7 and Alg13 add phospho-GlcNAc to Dol phosphate, and Alg1, Alg2, Alg11, Alg3, and Alg12 add the first, second, third, fourth, and fifth phospho-Man residues, respectively
[22][23][22,23]. The precursor is then flipped into the ER lumen in a process that involves the
RFT1 product
[17]. Within the ER lumen, the mannosyltransferases Alg3, Alg9, and Alg12 add four more mannose residues to the lipid-linked precursor, using Dol-P-Man as the sugar donor, and then Alg6, Alg8, and Alg10 add three glucose (Glc) units to generate the final glycan precursor Dol-PP-GlcNAc
2Man
9Glc
3 [2][13][2,13].
Once the precursor is assembled, it is transferred to the nascent protein by the oligosaccharyltransferase enzymatic complex
[13][24][13,24].
C. albicans encodes all the subunit orthologs found in the
S. cerevisiae oligosaccharyltransferase enzymatic complex, which is composed of nine different transmembrane subunits: Wbp1, Swp1, Stt3, Ost1, Ost2, Ost3, Ost4, Ost5, and Ost6, where Stt3 is the catalytic subunit
[13][25][13,25].
2.1.2. Modification of the N-Linked Glycan Core by Glycosidases and Transferases
Once the oligosaccharide has been transferred to the nascent protein, it is processed by ER α-glycosidases: α-glucosidases I and II, and α-1,2-mannosidase
[18][26][18,26]. α-Glucosidases I and II remove the terminal α-1,2-linked and two remaining α-1,3-linked glucose residues, respectively, and mannosidase generates the isomer Man
8GlcNAc
2 [24]. In
C. albicans,
CWH41 encodes α-glucosidase I,
ROT2 and
GTB1 encode the heterodimeric α-glucosidase II, and
MNS1 encodes the ER mannosidase
[3][26][3,26].
Following the glycosylation steps in the ER,
N-linked glycans are elongated by Golgi mannosyltransferases, which require GDP-Man as a sugar donor, and the transport of this compound into the lumen of the Golgi apparatus is mediated by a specific nucleotide sugar transporter encoded by
VRG4 [27]. The α-1,6-mannosyltransferase Och1 starts the addition of an α-1,6-mannose unit to the backbone of the
N-linked glycan outer chain (
Figure 1)
[28]. Subsequently, the α1,6-mannose backbone is extended by the sequential action of the mannan polymerase I (Mnn9 and Van1) and mannan polymerase II (Mnn9, Anp1, Mnn10, Mnn11, and Hoc1) enzyme complexes
[13][24][28][13,24,28]. This α-1,6-mannose backbone is branched with α-1,2-oligomannans by the action of mannosyltransferases Mnt3, Mnt4, Mnt5, Mnn2, and Mnn5, and this may be capped with terminal α-1,3-mannoses by the action of the mannosyltransferase Mnn1 (
Figure 1)
[13]. In
C. albicans, it is frequently found that the α-1,2-mannose branches are further β-mannosylated, a mechanism that is carried out by the
BMT gene family
[29]. Additionally, in this organism, the
N-linked glycans can be modified by mannosyl-phosphate moieties, named phosphomannans, which can work as molecular scaffolds to synthesize α-1,2-mannoligosaccharides
[24], in a process that involves the
MNN4-like gene family and the mannosyltransferases Mnt3 and Mnt5
[30][31][30,31].
2.2. The O-Linked Glycosylation Pathway
In
C. albicans, the
O-linked glycans are linear oligosaccharides of one to seven α-1,2-linked mannose residues
[32]. The addition of α-linked mannose residues to the serine or threonine residues is initiated in the ER lumen, using Dol-P-Man as a sugar donor, in a reaction catalyzed by any of the five members of the protein mannosyltransferase (
PMT) gene family
[13][33][13,33]. Then, glycoproteins are transported to the Golgi complex to start the addition of additional mannose residues by the action of Golgi α-1,2-mannosyltransferases encoded by
MNT1 and
MNT2 [32][34][32,34]. These are GDP-mannose-dependent mannosyltransferases that may have redundant activities but with a preference to add particular mannose residues to the
O-linked glycans: Mnt1 adds the second mannose, whereas Mnt2 adds the third mannose unit during the elongation step (
Figure 1)
[32]. These
O-linked glycans can also be phosphomannosylated by Mnt3 and Mnt5
[31].
2.3. Glycosylphosphatidylinositol Anchor Synthesis
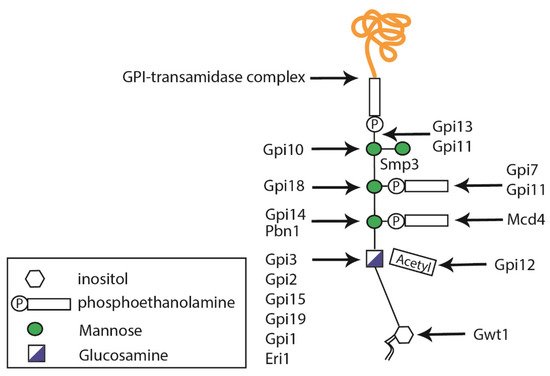
GPI anchors are structurally complex glycophospholipids that are post-translationally attached to the
C-terminal end of proteins containing the appropriate GPI signal sequence
[35]. The first step in GPI biosynthesis takes place in the ER, where a phosphatidylinositol receives GlcNAc from UDP–GlcNAc, in a reaction catalyzed by a multi-subunit GPI-N-acetylglucosaminyltransferase (GPI-GnT) complex
[6], whose catalytic subunit is encoded by
GPI19 (
Figure 2)
[13][36][13,36]. Then, the inositol-acyl group is removed during GPI maturation, triple mannosylated, and decorated with two–three phosphoethanolamine groups at the different mannoses (
Figure 2)
[6]. In fungi, the addition of a fourth mannose residue is required and catalyzed by the mannosyltransferase Smp3
[37][38][37,38]. The complete GPI-anchored protein is formed once the anchor is attached to a new translocated protein in the ER lumen, via the GPI transamidase complex composed of Gaa1, Gpi8, and Gpi16
[39], and is then transported via the secretory pathway to the cell wall or plasma membrane.
Figure 2. Schematic diagram representing glycosylphosphatidylinositol (GPI) anchor synthesis. GPIs are composed of an inositol molecule that is modified in the endoplasmic reticulum. The Smp3 protein is responsible for adding the fourth mannose residue, which is important for protein transfer.
3. Protein Glycosylation in Other Medically Relevant Fungal Pathogens
In general terms, the dissected models of
C. albicans protein glycosylation can be applied to other fungal species, although species-specific glycans have been isolated from clinically relevant yeasts and molds.
In
A. fumigatus, the cell wall contains galactomannan, which is a linear polysaccharide of α-1,2- and α-1,6-mannose units and branched with β-1,5- and β-1,6-galactofuranose residues
[40], and is covalently linked to
N-linked and
O-linked glycans, β-(1,3)-glucans, and cell membrane GPI anchors
[40][41][40,41]. Galactofuranose-containing glycans are peculiar and not synthesized by the human host. The first step in their synthesis involves the generation of UDP-galactofuranose, by the action of a cytosolic UDP-galactopyranose mutase, encoded by
ugm1 [42]; then, a UDP-galactofuranose transporter, GlfB, imports this activated sugar into the Golgi lumen, where the galactofuranosyltransferase GfsA binds this sugar moiety to glycans
[43][44][45][43,44,45]. The mannose-rich core of galactomannan is also synthesized in the Golgi complex, and the α-1,2-mannosyltransferases ktr4 and ktr7 have been implicated in this biosynthetic process
[46][47][46,47]. Since a
glfBΔ mutant did not show galactosylated high-mannose
N-linked glycans, it was proposed that this transport is required for galactofuranosylation of
A. fumigatus N-linked glycans
[45]. Even though it is not covalently linked to proteins by the canonical protein glycosylation pathways, it is worthy of mention that galactosaminogalactan, a galactopyranose-containing glycan, is in the surface of
A. fumigatus mycelia
[48]. In addition to galactopyranose, it also contains galactosamine and N-acetylgalactosamine bound via α-1,4-linkages
[48].
The generation of an
A. fumigatus undecuple mutant, lacking
och1-1,
och1-2,
och1-3,
och1-4,
mnn9,
van1,
anp1,
mnn10,
mnn11,
mnn2, and
mnn5, putative orthologs of genes encoding for α-1,2- and α-1,6-mannosyltransferases, did not show a reduction in the hyphal wall mannan content, but this was affected when the conidial cell wall was analyzed
[49], suggesting that canonical genes, previously assigned to the protein glycosylation pathways, may have different functions in molds, and that their function is tightly regulated by cell morphology and likely by environmental cues.
Different
N-linked and
O-linked glycans have also been reported in
Cryptococcus spp. There is strong evidence suggesting that the
N-linked glycan core is similarly synthesized in both
C. albicans and
C. neoformans, but the enzymes involved in the synthesis of the
N-linked outer chain seem to be absent from the
C. neoformans genome
[50], suggesting no high-mannose
N-linked glycans are synthesized in this organism. However, they may contain sialic acid or xylose
[51][52][51,52]. Different types of
O-linked glycans have been characterized in
Cryptococcus laurentii: linear α-1,2-mannotriose and oligomannose chains modified with xylose, and extended chains of α-1,6-galactose linked to a single mannose residue
[53]. In the case of
C. neoformans, the most abundant
O-linked glycans are α-1,2-mannans but connected by an α-1,6-mannose at the third position of the glycan
[54]. As a minority, xylose-modified
O-linked glycans are also present
[54]. Analysis of the biosynthetic machinery behind these structures indicated that the α-1,2-mannosyltransferase Ktr3 is in charge of adding the second mannose residue, and the α-1,6-mannosyltransferases Hoc1 and Hoc3 add the third mannose unit to xylosylated and non-xylosylated
O-linked glycans, respectively
[54]. Finally, xylose is transferred to glycans from UDP-xylose, which is synthesized by the
UXS1 product
[54], and
XPT1 that codes for a Golgi-resident xylosyltransferase
[55].
In
Sporothrix schenckii, rhamnose-containing
N- and
O-linked glycans have been reported and contain terminal α-1,2-, α-1,3-, or α-1,4-rhamnose units
[56]. In addition,
O-linked glycans mainly contain glucuronic acid, which may be mono- or bi-rhamnosylated
[56]. Protein rhamnosylation depends on the presence of
RmlD, a gene encoding for an epimerase/reductase essential for the elaboration of UDP-rhamnose, the sugar donor in this post-translational modification
[57]. Moreover, sialic acid- and galactose-containing
N-linked glycans have been reported in the
S. schenckii cell wall
[58].