Persufflation (PSF) utilises the organ's own vascular network to provide oxygen to the organ tissue and maintain metabolism during preservation to avoid hypoxic damage. This method discovered in the early 20th century has shown promise in providing both longer-term preservation and organ reconditioning capabilities for multiple organs including the liver, kidneys, and pancreas.
- gaseous oxygen perfusion
- hypothermic reconditioning
- organ preservation
- organ perfusion
- persufflation
- VSOP
1. Introduction
Currently, the most commonly used method of preservation, static cold storage (SCS), provides a simple and cost-effective method of preserving organs. This technique works by slowing the metabolism of the organ using specialised preservation solutions. However, lack of oxygen for extended periods leads to deleterious effects which may impact on transplantation outcomes. Whilst this technique is acceptable for standard criteria donors over shorter preservation times, it has been shown to be incapable of preserving them for longer durations. This technique’s shortcomings become more prevalent when using extended-criteria donor organs. Due to the lack of active oxygen supply, there is an increase in risk with extended preservation times that can lead to hypoxia and the damage associated with it, especially with large solid organs [8,9][1][2]. This has driven the need for improved techniques that can cater for these requirements. So-called ’active’ preservation techniques, which typically perfuse fluids through the existing vasculature, have shown to be able to do just that. Various active preservation techniques have been investigated that have been shown to be better at providing oxygen directly to the organ and thus providing extended preservation times without degradation in organ viability. Such techniques include hypothermic machine perfusion (HMP) and normothermic machine perfusion (NMP) which perfuse the organ using specialised preservation solution, or persufflation (PSF) which uses humidified oxygen gas directly as the perfusate.
PSF was first described in the early 20th century when it was discovered by R Magus, which went unused for several decades due to a lack of possible applications [10][3]. This technique would see a resurgence in the late 1950’s after the first successful transplantation in humans was performed; however, its usage as a preservation technique was overshadowed by the discovery and adoption of SCS in the 1960’s [11][4]. Interest in PSF remained prevalent in the following decades with two different approaches, retrograde and anterograde, being described in the 1970’s [12][5]. In recent years, PSF has regained the interest of the scientific community for its ability to aptly provide oxygenation to larger organs, its relative ease of use, and simplicity when compared to other advanced preservation techniques [13][6]. PSF has shown promise in improving the durations an organ can be safely preserved for, whilst also maintaining organ quality to help improve the outcome of the transplantation.
2. Persufflation
2.1. What Is Persufflation?
Organ preservation and transplantation presents various logistical and technical challenges. Organs are often required to be transported great distances and may face local competition from operating theatres necessitating extended preservation. This frequently leads to operations being performed out-of-hours by tired transplant teams, which has been shown to lead to a higher rate of surgical complications [14][7].
The most common form of organ preservation, SCS, relies solely on reducing metabolism whilst osmotically balancing the extracellular space to match what cells experience intracellularly during hypothermic storage. While this can provide adequate preservation from traditional donor organs exposed to brief periods of cold ischaemia, it can manifest several changes over time, which may damage the organ either during preservation itself or following reperfusion leading to what is known as Ischaemia Reperfusion Injury (IRI) [15,16][8][9]. These changes damage organs and may lead to dangerous scenarios if transplanted; resulting in an often overly cautious approach and unfortunately resulting in wastage of transplantable organs. To address this, a variety of preservation technologies have been investigated in recent years.
PSF is one such technology, which aims to restore tissue oxygenation by providing maintenance of bioenergetic status; ameliorating tissue degradation due to hypoxic exposure during ischaemia. This enables a longer period of possible preservation facilitating more successful transplants with better posttransplant function. For PSF, the organ or tissue that is being preserved, is cannulated and connected to a device that perfuses oxygen gas directly into the vasculature of the graft. Multiple studies have demonstrated that, for larger organs, this method provides superior oxygenation to the organs when compared to alternatives such as SCS [17,18][10][11]. This has been shown to extend the preservation time as well as providing some restorative benefits to marginal organ grafts.
Hypothermic reconditioning (HR) is also a key interest of PSF studies. This being the process of applying PSF after a period of SCS to reverse ischaemia associated alterations, reduce the risk of IRI, and permanent tissue damage [19][12].
PSF is typically performed in either of two ways as can be seen in Figure 1. Retrograde Persufflation (R-PSF), is the process of cannulating the vein of the organ to pass the gaseous oxygen through. This method requires that the organ is punctured using multiple pinpricks to gradually let the oxygen escape from the organ. Using a high concentration of oxygen (>95%), gas is perfused through the venous system in a reversed manner to normal physiological flow. This version requires lower pressures to perfuse oxygen through the organ leading research to suggest that it has no adverse effect on venous endothelium [20][13]. On the other hand, Anterograde Persufflation (A-PSF), works like R-PSF with the distinction that the flow is reversed to mimic the natural flow of blood in the body, typically entering via an artery before traversing the capillary bed and draining via the vein. Studies have shown that A-PSF has demonstrated the ability to provide more efficient gas exchange when compared to R-PSF due to the higher surface area provided by the capillary bed and therefore lower concentrations of oxygen (40%) are used to avoid damaging the graft tissue.
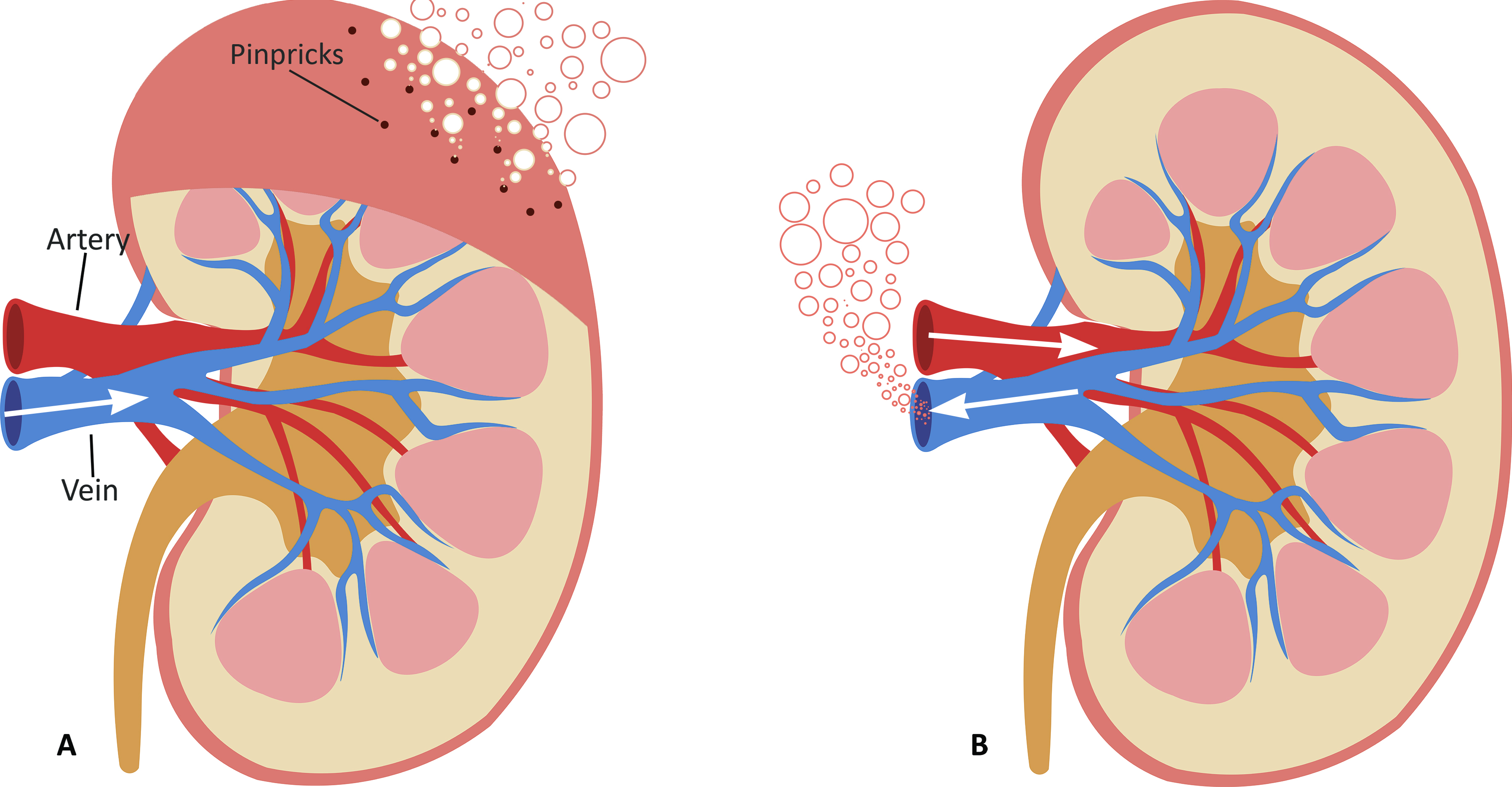
Figure 1. Cross-sectional diagram of the kidney being persufflated using two different approaches: (A) Retrograde Persufflation (R-PSF); with the gas being perfused through the vein and bubbling out through the pinpricks. (B) Anterograde Persufflation (A-PSF); with the gas being perfused through the artery and exiting through the vein.
2.2. History (1902–2005)
PSF was discovered in 1902, when Rudolf Magnus unexpectedly observed a feline heart rhythmically contract, while it was perfused by oxygen gas at sub-normothermic temperatures. Whilst originally perfusing the heart with defibrinated blood, which was being oxygenated by compressed oxygen gas, an over-pressurisation of the blood reservoir caused the oxygen gas to be accidentality perfused directly into the heart. Encouraged by these observations, Magnus continued studying this phenomenon and discovered that it was possible to keep the heart beating for over 1 h using this method [10]. PSF would go largely unstudied for several decades until human organ transplantation was shown to be possible [21].
New techniques for organ preservation were being investigated as means to better preserve organs for a longer time. Between the late 1950’s and the late 1960’s, most studies centred around the heart and investigating its cardiac activity during PSF and post-reperfusion [22,23,24,25]. In the early 1970’s focus shifted onto kidney preservation [26]. Isselhard conducted various studies on the effects of PSF on canine kidneys [12,26,27]. Amongst these studies, several compared the usage of R-PSF against A-PSF and analysed the results to determine the efficacy of each method and which yielded the best results, whilst using pure oxygen as the perfusate. At the time it was determined that R-PSF produced the better result [28,29].
A key pilot study at Cambridge University was performed in 1989 by Rolles et al. who investigated the use of R-PSF on human kidneys. They surmised that even given the slight complexity added due to the use of PSF, it was acceptable to use as it improved both initial function and graft quality compared to SCS [30]. Studies investigating kidneys mainly using R-PSF remained prevalent throughout the following years.
In the 1990’s, focus shifted to the liver. Initially, the first liver PSF study was conducted in 1980 by Fischer, however it was not until the mid to late 90’s when Minor and colleagues published a series of papers studying the effect of PSF primarily on rat liver grafts [20,31,32,33,34]. These studies detailed the efficacy of the method as a means of preserving liver grafts and understanding its metabolic profile during reperfusion. Due to the size of livers, one key aim of these was to investigate the level of oxygenation achieved with the organ both during preservation and afterwards.
Around the turn of the century, PSF studies continued primarily using porcine grafts. Studies on the heart by Kuhn-Regnier and Fischer investigated the effect of A-PSF and its impact on cardiac function and endothelial status [35,36]. Similar studies were conducted by Treckmann using kidneys and Saad and Lauschke for livers [37,38,39]. Several of these studies used DCD organs; which has since become a growing trend in the field of PSF. These organs require greater care during preservation due to their poorer quality and PSF has been investigated as means to improve their efficacy for transplantation.
3. Recent Works (Post–2005)
In recent years, PSF development has focused mainly on three different organs, these being the liver, kidney, and pancreas. Using varying animal models, researchers have attempted to assess the different aspects of PSF and its effects.
3.1. Liver
A variety of studies were conducted on the liver, such as those that aim to investigate the effects and the parameters of the technique as well as its usage on DCD livers. Most notably, researchers such as Minor, Koetting, and Stegemann used various models to identify the optimal time, concentration of oxygen, and sequence of using PSF to obtain the best results [13,45,59][6][33][34].
Beyond this, the usage of adjuvant approaches during preservation, such as using antioxidant agents to provide additional protection to the organ, has been explored primarily in murine models. Several groups attempted to mix the oxygen with nitric oxide which led to improved results in terms of graft quality and viability [49,51,52,54,56][34][35][36][37][38]. In a similar fashion, Koetting et al. used carbon monoxide to suppress early inflammatory reaction and ischemia-reperfusion injury. The treated livers exhibited a significant improvement in liver function and graft tissue integrity compared to SCS but similar results to normal PSF [42,44][39][40].
The application of PSF extended to other types of marginal organs such as steatotic or hypovolemic shocked organs. In a study by Jafari et al., PSF was used on hypovolemic shocked livers to preserve and hypothermically recondition them which ultimately produced no favourable results as the organs continued to deteriorate[57] [41]. Steatotic livers showed significant improvement when preserved with PSF supplemented with high-dose reduced-glutathione[43] [42]. Human mildly steatotic livers also benefited significantly from the usage of PSF as described in a study by Khorsandi et al. [55][43].
Clinical application of PSF was first attempted by Treckmann et al. in 2008. Rejected marginal livers with various levels of WIT were treated with PSF and transplanted into patients with low MELD scores. The study analysed functional markers of the liver pre and post-transplantation. The results showed that the liver showed significant improvement in these measurements as well as exhibiting good primary graft function [41][44].
More recently, a clinical trial was conducted for the usage of PSF on livers. Originally designed by Minor et al. in 2011, the results of the 116 patient trial were published by Gallinat et al. in 2019 [47,58][45]. The cohort of patients (who had low MELD scores) was split into 2 groups, one receiving marginal livers treated with 2 hours of PSF and the other being the control group, receiving untreated marginal livers. The results obtained for the one to five-year survival rates of both groups were similarly high, with the PSF group showing improved results in the older age group (70+) [58][45].
3.2. Kidney
Similarly to the liver, many studies in this period for the kidney explored the efficacy of the method both for preservation as well as a hypothermic reconditioning tool.
Treckmann et al. used PSF on kidneys that had experienced varying durations of WIT, discovering that its benefits were limited to the organs that had been exposed to 1 hour or less of WIT [39][32]. Minor et al. investigated HR after longer-term preservation with durations up to 18 hours, demonstrating the viability of this technique to ‘resuscitate’ organs that have exceeded the clinical maximum for SCS [60,63][46][47].
This period saw several studies that compared various preservation techniques to PSF, including studies by Teckmann et al. in 2009, Kalenski et al. in 2016 as well as Molacek et al. and Min in 2018 [18,65,66,67][11][48][49][50]. Whilst using different parameters, the common result obtained was that PSF is mostly comparable to other active techniques such as HMP whilst being superior to SCS.
3.3. Pancreas
For pancreas preservation, most recent works centered around the effects of PSF and comparison to other techniques, mainly in human or porcine models.
Efficacy of PSF was investigated in 2014 by Reddy et al. on DCD rat pancreta. They demonstrated that, whilst the resultant organs were of poorer quality when compared to non-DCD grafts, they were significantly better than those preserved solely with SCS [68][51].
Scott et al. published several works comparing PSF to an advanced variant of SCS called the two-layer method (TLM). In these works it was shown that PSF was able to better oxygenate the pancreas grafts and maintain a high level of certain functional markers such as ATP [17, 70][10][52]. They later reported improved islet isolation yield and quality in the PSF group when compared to TLM [71][53]. On the topic islets, Kelly et al. conducted a study in 2019, investigating the effects of preservation on them. Whilst the PSF group underwent longer periods of preservation than their SCS counterpart, they showed no adverse effects. The study concludes by discussing the negative effects that prolonged preservation using SCS has on the islet and insulin secretion [54][55]. [72,73].
3.4. Other Applications
Historically, the heart was a mainstay of PSF studies, however, in recent times interest seems to have subsided. Whilst we are aware of several abstracts being presented during the past 15 years, we were unable to find any publications to report in this review.
We are also aware of several abstracts investigating the use of PSF for composite tissue preservation such as for limbs. However, we were similarly unable to find publication to report in this review. These abstracts demonstrate that there is some interest in other applications and we look forward to seeing more publications emerging moving forward.
We are presently unaware of any other applications of PSF beyond those mentioned above.
References
- Iwanaga, Y.; Sutherland, D.E.; Harmon, J.V.; Papas, K.K. Pancreas preservation for pancreas and islet transplantation. Curr. Opin. Organ. Transplant. 2008, 13, 445–451.
- Taylor, M.J.; Baicu, S.C. Current state of hypothermic machine perfusion preservation of organs: The clinical perspective. Cryobiology 2010, 60, 20–35.
- Magnus, R. Thätigkeit des überlebenden Säugethierherzens bei Durchströmung mit Gasen. Arch. Exp. Pathol. Pharmakol. 1902, 47, 200–208.
- Barker, C.F.; Markmann, J.F. Historical overview of transplantation. Cold. Spring Harb. Perspect. Med. 2013, 3, a014977.
- Isselhard, W.; Denecke, H.; Witte, J.; Berger, M.; Fischer, J.H. Renal function after hypothermic kidney ischemia with orthograde and retrograde O2-persulflation in situ. Res. Exp. Med. 1972, 157, 231–234.
- Stegemann, J.; Minor, T. Energy charge restoration, mitochondrial protection and reversal of preservation induced liver injury by hypothermic oxygenation prior to reperfusion. Cryobiology 2009, 58, 331–336.
- Lonze, B.E.; Parsikia, A.; Feyssa, E.L.; Khanmoradi, K.; Araya, V.R.; Zaki, R.F.; Segev, D.L.; Ortiz, J.A. Operative start times and complications after liver transplantation. Am. J. Transplant. 2010, 10, 1842–1849.
- Minor, T.; von Horn, C. Rewarming Injury after Cold Preservation. Int. J. Mol. Sci. 2019, 20, 2059.
- Wei, J.; Wang, Y.; Zhang, J.; Wang, L.; Fu, L.; Cha, B.J.; Buggs, J.; Liu, R. A mouse model of renal ischemia-reperfusion injury solely induced by cold ischemia. Am. J. Physiol. Ren. Physiol. 2019, 317, F616–F622.
- Scott, W.E.; Weegman, B.P.; Ferrer-Fabrega, J.; Stein, S.A.; Anazawa, T.; Kirchner, V.A.; Rizzari, M.D.; Stone, J.; Matsumoto, S.; Hammer, B.E.; et al. Pancreas oxygen persufflation increases ATP levels as shown by nuclear magnetic resonance. Transplant. Proc. 2010, 42, 2011–2015.
- Treckmann, J.; Nagelschmidt, M.; Minor, T.; Saner, F.; Saad, S.; Paul, A. Function and quality of kidneys after cold storage, machine perfusion, or retrograde oxygen persufflation: Results from a porcine autotransplantation model. Cryobiology 2009, 59, 19–23.
- Minor, T.; Paul, A. Hypothermic reconditioning in organ transplantation. Curr. Opin. Organ. Transplant. 2013, 18, 161–167.
- Fischer, J.H. Hypothermic liver preservation using different flush solutions and retrograde oxygen persufflation technique. Eur. Surg. Res. 1980, 12, 19–20.
- Merrill, J.P.; Murray, J.E.; Harrison, J.H.; Guild, W.R. Successful homotransplantation of the human kidney between identical twins. J. Am. Med. Assoc. 1956, 160, 277–282.
- Arnold, G.; Müller-Ruchholtz, E.R.; Lochner, W. The prolongation of the survival time of ischemic hearts by perfusing the coronary arteries with gaseous oxygen. Arztl. Forsch. 1968, 22, 257–264.
- Burns, B.D.; Robson, J.G.; Smith, G.K. The survival of mammalian tissues perfused with intravascular gas mixtures of oxygen and carbon dioxide. Can. J. Biochem. Physiol. 1958, 36, 499–504.
- Sabiston, D.C.; Talbert, J.L.; Riley, L.H.; Blalock, A. Maintenance of the heart beat by perfusion of the coronary circulation with gaseous oxygen. Ann. Surg. 1959, 150, 361–370.
- Camishion, R.C.; Davies, A.L.; Tokunaga, K.; Solit, R.W. Retrograde perfusion of the coronary arteries with gaseous oxygen cardiopulmonary bypass. Surgery 1966, 59, 145–154.
- Isselhard, W.; Berger, M.; Denecke, H.; Witte, J.; Fischer, J.H.; Molzberger, H. Metabolism of canine kidneys in anaerobic ischemia and in aerobic ischemia by persufflation with gaseous oxygen. Pflugers Arch. 1972, 337, 87–106.
- Sachweh, D.; Isselhard, W.; Dennecke, H.; Stelter, W.J.; Berger, M.; Lauschke, H.; Eigler, W.F. Short time kidney preservation by hypothermic oxygen persufflation. Bull. Soc. Int. Chir. 1972, 31, 258–263.
- Isselhard, W.; Denecke, H.; Stelter, W.; Berger, M.; Sachweh, D.; Witte, J.; Fischer, J.H. Function and metabolism of canine kidneys after aerobic ischemia by orthograde persufflation with gaseous oxygen. Res. Exp. Med. 1973, 159, 288–297.
- Isselhard, W.; Witte, J.; Denecke, H.; Berger, M.; Fischer, J.H.; Molzberger, H. Function and metabolism of canine kidneys after aerobic ischemia by retrograde persufflation with gaseous oxygen. Res. Exp. Med. 1974, 164, 35–44.
- Rolles, K.; Foreman, J.; Pegg, D.E. A pilot clinical study of retrograde oxygen persufflation in renal preservation. Transplantation 1989, 48, 339–342.
- Minor, T.; Isselhard, W. Synthesis of high energy phosphates during cold ischemic rat liver preservation with gaseous oxygen insufflation. Transplantation 1996, 61, 20–22.
- Minor, T.; Isselhard, W.; Klauke, H. Reduction in nonparenchymal cell injury and vascular endothelial dysfunction after cold preservation of the liver by gaseous oxygen. Transplant. Int. 1996, 9 (Suppl. 1), S425–S428.
- Minor, T.; Klauke, H.; Isselhard, W. Improved preservation of the small bowel by luminal gas oxygenation: Energetic status during ischemia and functional integrity upon reperfusion. Transplant. Proc. 1997, 29, 2994–2996.
- Minor, T.; Saad, S.; Nagelschmidt, M.; Kötting, M.; Fu, Z.; Paul, A.; Isselhard, W. Successful transplantation of porcine livers after warm ischemic insult in situ and cold preservation including postconditioning with gaseous oxygen. Transplantation 1998, 65, 1262–1264.
- Kuhn-Régnier, F.; Bloch, W.; Tsimpoulis, I.; Reismann, M.; Dagktekin, O.; Jeschkeit-Schubbert, S.; Funcke, C.; Fries, J.W.; Addicks, K.; de Vivie, E.R.; et al. Coronary oxygen persufflation for heart preservation in pigs: Analyses of endothelium and myocytes. Transplantation 2004, 77, 28–35.
- Fischer, J.H.; Funcke, C.; Yotsumoto, G.; Jeschkeit-Schubbert, S.; Kuhn-Régnier, F. Maintenance of physiological coronary endothelial function after 3.3 h of hypothermic oxygen persufflation preservation and orthotopic transplantation of non-heart-beating donor hearts. Eur. J. Cardiothorac. Surg. 2004, 25, 98–104.
- Lauschke, H.; Kötting, M.; Akbar, S.; Minor, T. Use of taurine as antioxidant in resuscitating livers from non-heart-beating donors by gaseous oxygen persufflation. J. Investig. Surg. 2003, 16, 7–11.
- Saad, S.; Minor, T.; Kötting, M.; Fu, Z.X.; Hagn, U.; Paul, A.; Nagelschmidt, M. Extension of ischemic tolerance of porcine livers by cold preservation including postconditioning with gaseous oxygen. Transplantation 2001, 71, 498–502.
- Treckmann, J.W.; Paul, A.; Saad, S.; Hoffmann, J.; Waldmann, K.H.; Broelsch, C.E.; Nagelschmidt, M. Primary organ function of warm ischaemically damaged porcine kidneys after retrograde oxygen persufflation. Nephrol. Dial. Transplant. 2006, 21, 1803–1808.
- Koetting, M.; Lüer, B.; Efferz, P.; Paul, A.; Minor, T. Optimal time for hypothermic reconditioning of liver grafts by venous systemic oxygen persufflation in a large animal model. Transplantation 2011, 91, 42–47.
- Srinivasan, P.K.; Yagi, S.; Doorschodt, B.; Nagai, K.; Afify, M.; Uemoto, S.; Tolba, R. Impact of venous systemic oxygen persufflation supplemented with nitric oxide gas on cold-stored, warm ischemia-damaged experimental liver grafts. Liver Transplant. 2012, 18, 219–225.
- Nagai, K.; Yagi, S.; Afify, M.; Bleilevens, C.; Uemoto, S.; Tolba, R.H. Impact of venous-systemic oxygen persufflation with nitric oxide gas on steatotic grafts after partial orthotopic liver transplantation in rats. Transplantation 2013, 95, 78–84.
- Yagi, S.; Nagai, K.; Kadaba, P.; Afify, M.; Teramukai, S.; Uemoto, S.; Tolba, R.H. A novel organ preservation for small partial liver transplantations in rats: Venous systemic oxygen persufflation with nitric oxide gas. Am. J. Transplant. 2013, 13, 222–228.
- Kageyama, S.; Yagi, S.; Tanaka, H.; Saito, S.; Nagai, K.; Hata, K.; Fujimoto, Y.; Ogura, Y.; Tolba, R.; Shinji, U. Graft reconditioning with nitric oxide gas in rat liver transplantation from cardiac death donors. Transplantation 2014, 97, 618–625.
- Porschen, A.; Kadaba Srinivasan, P.; Iwasaki, J.; Afify, M.; Tolba, R.H. Optimal Timing for Venous Systemic Oxygen Persufflation Supplemented with Nitric Oxide Gas in Cold-Stored, Warm Ischemia-Damaged Experimental Liver Grafts. Eur. Surg. Res. 2016, 57, 100–110.
- Koetting, M.; Dombrowski, F.; Minor, T. No synergistic effect of carbon monoxide and oxygen during static gaseous persufflation preservation of DCD livers. J. Surg. Res. 2011, 171, 859–864.
- Koetting, M.; Leuvenink, H.; Dombrowski, F.; Minor, T. Gaseous persufflation with carbon monoxide during ischemia protects the isolated liver and enhances energetic recovery. Cryobiology 2010, 61, 33–37.
- Jafari, A.; Matthaei, H.; Branchi, V.; Bölke, E.; Tolba, R.H.; Kalff, J.C.; Manekeller, S. Donor liver quality after hypovolemic shock and venous systemic oxygen persufflation in an experimental animal model. Eur. J. Med. Res. 2018, 23, 51.
- Ye, S.; Dong, J.; Han, B. Protective effect of reduced glutathione and venous systemic oxygen persufflation on rat steatotic graft following liver transplantation. J. Surg. Res. 2010, 158, 138–146.
- Khorsandi, S.E.; Jitraruch, S.; Fairbanks, L.; Cotoi, C.; Jassem, W.; Vilca-Melendez, H.; Prachalias, A.; Dhawan, A.; Heaton, N.; Srinivasan, P. The effect of anterograde persufflation on energy charge and hepatocyte function in donation after cardiac death livers unsuitable for transplant. Liver Transplant. 2014, 20, 698–704.
- Treckmann, J.; Minor, T.; Saad, S.; Ozcelik, A.; Malagó, M.; Broelsch, C.E.; Paul, A. Retrograde oxygen persufflation preservation of human livers: A pilot study. Liver Transplant. 2008, 14, 358–364.
- Gallinat, A.; Hoyer, D.P.; Sotiropoulos, G.; Treckmann, J.; Benkoe, T.; Belker, J.; Saner, F.; Paul, A.; Minor, T. Oxygen Persufflation in Liver Transplantation Results of a Randomized Controlled Trial. Bioengineering 2019, 6, 35.
- de Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Supercooling extends preservation time of human livers. Nat. Biotechnol. 2019, 37, 1131–1136.
- Kalenski, J.; Mancina, E.; Paschenda, P.; Beckers, C.; Bleilevens, C.; Túthová, Ĺ.; Boor, P.; Gross, D.; Tolba, R.H.; Doorschodt, B.M. Comparison of Aerobic Preservation by Venous Systemic Oxygen Persufflation or Oxygenated Machine Perfusion of Warm-Ischemia-Damaged Porcine Kidneys. Eur. Surg. Res. 2016, 57, 10–21.
- Moláček, J.; Opatrný, V.; Matějka, R.; Baxa, J.; Třeška, V. Retrograde Oxygen Persufflation of Kidney—Experiment on an Animal. In Vivo 2016, 30, 801–805.
- Moláček, J.; Opatrný, V.; Třeška, V.; Matějka, R.; Hes, O. Options to improve the quality of kidney grafts from expanded criteria donors experimental study. Rozhl. Chir. 2018, 97, 193–201.
- Min, C.G. Evaluation of Persufflation and Cold Storage Preservation in Isolated Porcine Kidneys Using Novel Methods for Organ Quality Assessments. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 2018.
- Reddy, M.S.; Carter, N.; Cunningham, A.; Shaw, J.; Talbot, D. Portal Venous Oxygen Persufflation of the Donation after Cardiac Death pancreas in a rat model is superior to static cold storage and hypothermic machine perfusion. Transplant. Int. 2014, 27, 634–639.
- Scott, W.E., III. Application of NMR in the Characterization of Existing and Development of New Methods for Pancreas Preservation. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2012.
- Scott, W.E.; O’Brien, T.D.; Ferrer-Fabrega, J.; Avgoustiniatos, E.S.; Weegman, B.P.; Anazawa, T.; Matsumoto, S.; Kirchner, V.A.; Rizzari, M.D.; Murtaugh, M.P.; et al. Persufflation improves pancreas preservation when compared with the two-layer method. Transplant. Proc. 2010, 42, 2016–2019.
- Kelly, A.C.; Smith, K.E.; Purvis, W.G.; Min, C.G.; Weber, C.S.; Cooksey, A.M.; Hasilo, C.; Paraskevas, S.; Suszynski, T.M.; Weegman, B.P.; et al. Oxygen Perfusion (Persufflation) of Human Pancreata Enhances Insulin Secretion and Attenuates Islet Proinflammatory Signaling. Transplantation 2019, 103, 160–167.
- Hosgood, S.A.; Nicholson, M.L. Reducing Proinflammatory Signaling and Enhancing Insulin Secretion with the Application of Oxygen Persufflation in Human Pancreata. Transplantation 2019, 103, 13–14.
