Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Hamza Mechchate.
In Oulmes (Middle Atlas, Morocco), the local population uses C. ladanifer traditionally to treat various diseases and health issues due to its antioxidant, gastric, anti-inflammatory, antitumor, antimicrobial, and antiviral properties. This plant is usually harvested in May in this area (flowering time). The leaves of all Cistus species secrete essential oils. Essential oils from different species of medicinal plants have been documented to possess antimicrobial propriety with strong activity against Gram-negative and Gram-positive bacteria and also fungi.
- C. ladanifer var. maculatus Dun
- essential oil
- antimicrobial activity
- yeast
- mold
- chemical analysis
1. Introduction
Aromatic and medicinal plants (AMPs) have long been a part of man’s everyday existence for a variety of purposes. AMPs play a crucial and fundamental role in traditional medicine and play a very significant role in drug discovery [1]. Natural substances account for around 25% of all medicines available for the treatment of illnesses (plants, animals, bacteria, and fungi) [2]. Cistus ladanifer L. is one of the medicinal plants, belonging to the Cistaceae family, the latter represented by seven genera (Cistus, Fumane, Halimium, Tuberaria, Helianthemum, Hudsonia and Lechea), the genus Cistus alone encompass 16 species particularly distributed in the Mediterranean region [3,4][3][4], this kind is widespread in Portugal, Spain, Italy, Algeria, and Morocco [5]. The most common species are C. ladanifer (Gum Cistus), C. monspeliensis (Montpellier Cistus), C. salviifolius (Sage Cistus), C. laurifolius (Laurel Cistus), C. creticus (Cretan Cistus), and C. albidus (Cottony Cistus). C. ladanifer is represented in Morocco by two varieties that differ mainly by the color of the petals of flowers: C. ladanifer var. albiflorus Dun with completely white petals and C. ladanifer var. maculatus Dun with petals spotted with crimson. C. ladanifer is a much-exploited plant in Morocco, it is known as ‘Touzzalt’ in Amazigh, and in Arabic, it is called kastousse, Bouzegzaw, ftah, Targla, Touzzala’ [6]. It is a very fragrant spontaneous shrub; it has sticky branches up to 2 m tall, large white flowers (64 mm in diameter) that appear during spring (March–May) and have a three-day lifespan. Its seeds appear between July and October [7]. This species grows in very diverse climates; it is extremely resistant to cold stress, drought, and high temperatures [8].
In Oulmes (Middle Atlas, Morocco), the local population uses C. ladanifer traditionally to treat various diseases and health issues due to its antioxidant, gastric, anti-inflammatory, antitumor, antimicrobial, and antiviral properties [9,10,11][9][10][11]. This plant is usually harvested in May in this area (flowering time) [12]. The leaves of all Cistus species secrete essential oils [13]. Essential oils from different species of medicinal plants have been documented to possess antimicrobial propriety with strong activity against Gram-negative and Gram-positive bacteria and also fungi [14].
C. ladanifer essential oil is characterized by a large number of sesquiterpenes (viridiflorol and ledol) and monoterpenoids (bornyl acetate and pinocarveol) [15] that are maybe behind several reported activities such as analgesic, anti-inflammatory [16], antiplatelet [17], antioxidant [10[10][18],18], antidiarrheal, antispasmodic, anti-acid [19,20][19][20], antiulcer [21], antitumor and gastroprotective activities [22]. Sosa et al. confirmed that aqueous Cistus extract could be considered an inhibitor of calcium transport in skeletal muscles [23]. Belmoukhtar et al. and Aziz et al. showed that aqueous extract of C. Ladanifer has also been considered as a curative and preventive treatment for hypertension and the possibility of using it in the treatment of gastrointestinal disorders [20,24][20][24]. Amensour et al. reported that C. ladanifer is a source of natural antioxidants that can be exploited in the food industry given the high levels of existing flavonoids and phenolic compounds [25]. Finally, Andrade et al. and Barrajón-Catalánet al. have also reported its cytotoxic potential against several human cancer cells [10,11][10][11]. Due to bacterial and fungal resistance and the side effects of antibiotics, great interest has been given to biologically active molecules isolated from plant species [26], in particular, essential oils [19]. The objective of this work is to study for the first time the effect of C. ladanifer essential oil at its flourishing time (April) and the collection region (Oulmès region, Middle Atlas) on the chemical composition and antimicrobial effect of C. ladanifer var. maculatus Dun.
2. Current Results
2.1. C. Ladanifer Moisture Content and Its Essential Oil Yield Percentage
The moisture content of C. ladanifer essential oil was 13.2%. The yield extraction of the essential oil was around 0.21 ± 0.01%. These results were quite high compared to those obtained by several other researchers. In France, Robles reported a yield of 0.119 ± 0.016% [27]. In Algeria, Bechlaghem reported a yield of 0.08% [28]. Zidane et al. from Morocco obtained a yield of 0.14% [29]. Grech et al., also from Morocco found a yield of 0.3 to 0.4% in the northern region [30]. Thus, we find that the C. ladanifer essential oil yield, although highly variable, remains relatively low regardless of the region and the time of harvest.
2.2. Mineral (ash) and Organic Matter Content in the C. Ladanifer Essential Oil
The mineral content obtained was 3.7%, which was considered quite important. The organic content was around 1.35%. This variation can be explained by the mineral reserves of the soil, the efficiency of their root capture, and their movement towards the aerial organs of the C. ladanifer.
2.3. Refractive Index and Brix Index of the C. Ladanifer Essential Oil
The refractive index and Brix index are qualitative identification characteristics that may be used to evaluate the purity of essential oils [31]. Each substance has its specific refractive index. The purity of a product is determined by how near its refractive index is to the anticipated value. The refractive index of our studied essential oil is 1.45. Mrabet et al. reported a refractive index of 1.49 for the essential oil of C. ladanifer var maculatus from northern Morocco [32]. The refractive index values of C. ladanifer essential oil extracted by hydrodistillation are comparable to those of standards, indicating that our extracts are of excellent purity confirmed also with the low Brix index (1.33) which is an indicator of the concentration (%) of all solids dissolved in the essential oil (Table 1).
Table 1. Refractive index and Brix index of the C. ladanifer essential oil.
| Plant | Refractive Index | Brix Index |
|---|---|---|
| O | ||
| 0.64 | ||
| 1598 |
2.5.1. The Antibiotic Sensitivity Test
The antibiotic sensitivity profiles of the strains are developed according to the recommendations of the Committee on Antibiotic susceptibility of the French Society for Microbiology (CA-SFM) and presented in Table 3. From the table, it can be concluded that the strains of S. aureus and S. Typhi were sensitive to all antibiotics, while E. coli was only resistant to ticarcillin. At the same time, A. baumannii demonstrated complete resistance to all tested antibiotics. This may be due to the higher resistance of Gram-negative bacteria due to the complexity of their cell wall, containing a double membrane in opposition to the single glycoprotein/teichoic acid membrane of Gram-positive bacteria [35]
Table 3. The test for the sensitivity of bacterial strains to certain antibiotics.
| ATB | A. baumannii | ATB | S. aureus |
|---|---|---|---|
| C. ladanifer | L. essential oil | 1.45 | 1.33556 |
2.4. GC-SM Analysis of the Essential Oil of C. Ladanifer
The GC-MS analysis revealed the presence of 35 compounds in the essential oil of C. ladanifer (Figure 1). These compounds were divided into oxygenated sesquiterpenes (34.02%), oxygenated monoterpenes (33.14%), linear esters (10.38%), monoterpenes (9.11%), and sesquiterpenes (4.29%). The major constituents present in this EO were viridiflorol (17.64%), trans-pinocarveol (11.02%), bornylacetate (9.38%), and ledol (8.85%) (Table 2). The percentage of these constituents is higher than those found by Boukil et al. (oxygenated hydrocarbons (13.27%), oxygenated sesquiterpenes (2.57%), and monoterpenic ester (5.86%)) [19]. The same authors found that the main components from the fresh leaves of C. ladanifer were verticiol (18.16%), camphene (17.70%), n-butylcyclohexane (5.95%), and 3-carene (5.23%) [19]. These findings indicate that the time of harvest has a significant impact on the chemical composition obtained. The results of various chemical analysis studies on C. ladanifer essential oil carried out previously showed that the main constituents of C. ladanifer leaf essential oil from Northern Morocco were viridiflorol (19.6%), bornyl acetate (16.7%), and camphene (12.3%) [30]. Zidane et al. characterized C. ladanifer from Eastern Morocco and indicated the presence of camphene (15.5%), borneol (11.1%), 2,2,6-trimethylcyclohexanol (7.3%), 4-terpineol (6.3%), and α-pinene (4.2%) as the major compounds in the essential oil of this plant [29]. In Algeria, it was found that the main constituents of this oil were 5,7-di-epi-α-eudesmol (13.6%), borneol (12.5%), camphene (12.2%), δ-cadinene (7.6%), α-eudesmol (6.4%)%), 4-terpineol (5.7%) and α-pinene (4.2%) [28]. In France, Verdeguer et al. characterized the chemical composition of the oil extracted from the leaves and stems of C. ladanifer of Spanish origin but cultivated in Corsica by the presence of pinene (39%), viridiflorol (11.8%), ledol (3.3%) and bornyl acetate (3.1%) [33]. In Portugal, the chemical composition of C. ladanifer oil shows the presence of three sesquiterpenes alcohols, viridiflorol (13.6–17.4%), globulol (3.1–5.0%), and an unknown alcohol sesquiterpene (2.7–6.0%), as well as diterpene alcohol 15-nor-labdan-8-ol (1.7–5.2%) [34]. In Spain, the composition of the essential oil of C. ladanifer cultivated in central Spain, revealed its richness in oxygenated compounds, with trans-pinocarveol (20.00%), bornyl acetate (7.03%), and terpinen-4-ol (6.37%) as the main monoterpene compounds. Viridiflorol (13.59%) and ledol (4.36%) were the main constituents of the oxygenated sesquiterpene fraction. Large amounts of α-pinene (4.70%) were found in the hydrocarbon fractions. From this comparison, it seems that Moroccan C. ladanifer essential oil composition is closer to that of Corsica with Spanish origin. The chemical composition of C. ladanifer essential oil varies considerably depending on the source, plant material, and extraction method. As a result, based on the intended product during the exploitation of the species, a selection of organs, vegetative stage, and area proves to be extremely helpful in promoting the acquisition of very accurate chemotypes.
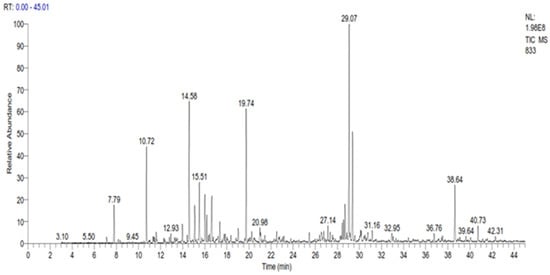

Figure 1. GC Chromatogram of the essential oil of C. ladanifer.
Table 2. The compounds identified in the essential oil of C. ladanifer after analysis by GC/MS on column DB-5.
| No. | Compounds | Formulas | Percentage | IK Calculated | IK (ADAMS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | C | 10 | H | 16 | 2.43 | 922 | 939 | |||||
| 2 | |||||||||||||
| TIC 75 μg | R | CIP 5 μg | S | ||||||||||
| p | -Cymene | ||||||||||||
| CEF 30 μg | R | C | 10 | H | 14 | 6.11 | 1004 | 1024 | |||||
| VAN 30 μg | S | 3 | ( | Z | )-vertocitral C | C | 9 | H | 14 | O | 0.63 | 1028 | 1080 |
| 4 | p | -Cymenene | C | 10 | H | 14 |
demonstrated full resistance to the tested antibiotics, it demonstrated an inhibition diameter of 35 ± 0.27 when the EO was used, indicating a good and promising effect. Our results are superior to those found by Benayad et al. (S. aureus (28 mm), A. Baumannii (24 mm), and E. coli (18 mm)) (plant harvested in May) [11]. Same note for results obtained by Boukil et al. (S. aureus (14 mm), and E. coli (9 mm)) (plant harvested in August) [19]. As an outcome, harvesting the plant at the flowering stage is correlated to a potent antibacterial power.


Figure 2. Bacterial strain growth inhibition zone of C. ladanifer essential oil.
Table 4. Diameter of the inhibition zone of C. ladanifer essential oil against the four pathogenic strains.
| S. aureus | E. coli | A. baumannii | S. Typhi | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C. ladanifer | 5 μL (EO) | 55 ± 0.22 | 42 ± 0.11 | 35 ± 0.27 | 30 ± 0.25 | ||||
| 0.57 | |||||||||
| 1065 | 1091 | ||||||||
| 5 | α-Campholenal | C | 10 | H | 16 | O | 1.34 | 1093 | 1126 |
The inhibition diameters were expressed in millimeters (mm): Mean ± SD.
2.5.3. Minimum Inhibitory Concentrations (MIC) and Minimum Bactericidal Concentrations (MBC)
During this investigation, the determination of the MIC was evaluated by assessing the inhibitory power of the plant’s essential oil at different concentrations against the selected bacteria (Table 5). In total accordance with the previous results obtained in the agar disk diffusion method and the observed inhibition zone, the results obtained indicated that the C. ladanifer essential oil MIC and MBC for all strains were 10 μL/mL. Regarding the MBC/MIC activity ratio, our results indicate that it was equal to 1 for all strains. This value allows us to affirm that the essential oil of C. ladanifer is bactericidal. This antimicrobial activity of this essential oil can only be explained by its chemical profile rich in 34.02% of oxygenated sesquiterpenes and oxygenated monoterpenes, and 33.14% of monoterpenes which are known as versatile anti-infective agents (antibacterial and antifungal) [36]. The structures of the functional groupings of the constituents of essential oils could play a crucial role in determining the antibacterial power of essential oils [37]. Nevertheless, minority compounds can interact directly, or in a synergistic or in an antagonistic way, to create a mixture with biological activity. Guinoiseau et al. [38], Rossi et al. [39], and Vieira et al. [40] demonstrated that essential oil from C. ladanifer has antimicrobial activity against Gram-positive and negative pathogens of clinical importance such as Staphylococcus aureus, E. coli, Streptococcus pneumonia, Pseudomonas aeruginosa, Enterobacter aerogenes, and Campylobacter jejuni.
Table 5. MIC and MBC of C. ladanifer essential oil against selected bacterial strains.
| Bacterial Strains | Concentrations (μL/mL) | DMSO 2 μL/mL |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 10 | 20 | 30 | 40 | 50 | MBC/MIC | |||
| S. aureus | MIC | + | − | − | − | − | − | 1 | + |
| MBC | + | − | − | − | − | − | + | ||
| Acinetobacter baumannii | MIC | + | − | − | − | − | − | 1 | + |
| MBC | + | − | − | − | − | − | + | ||
| E. coli | MIC | + | − | − | − | − | − | 1 | + |
| MBC | + | − | − | − | − | − | + | ||
| Salmonella Typhi | MIC | + | − | − | − | − | − | 1 | + |
| MBC | + | − | − | − | − | − | + | ||
+: Presence; −: Absence.
2.6. Antifungal Activity
The disc diffusion technique enabled us to demonstrate the antifungal activity of C. ladanifer essential oil against 10 different fungus strains (Figure 3). The antifungal effect of a volume of 5 μL, 10 μL, and 15 μL of C. ladanifer essential oil is presented in Table 6. The essential oil demonstrated a good inhibitory activity with a slight dose-dependent activity. Fluconazole used as a positive control presented the best inhibition zone diameters against all the selected strains. In relation to the essential oil activity, C. tropicalis and C. neoformans were the most sensitive with an inhibition zone of 13 mm, followed by R. rubra and Penicillium sp., which have the same inhibition diameter of 12 mm, then C. dubliniensis and C. glabrata with inhibition zones of 11 mm. No studies on the antifungal activity of C. ladanifer of our study area (Middle Atlas, Morocco) have been carried out, except for a few attempts that have been reported by Boukil et al. [16].
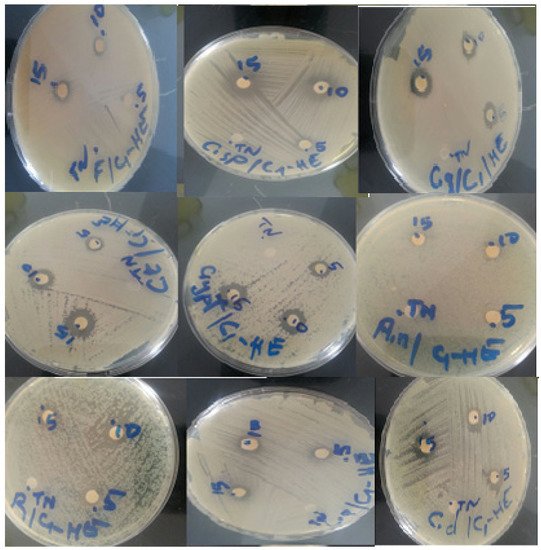

Figure 3. Fungal strains zone of inhibition diameters of essential oil of C. ladanifer.
Table 6. Values of the inhibition diameters zone of the essential oil of C. ladanifer.
| Fungal Strains | Growth Inhibition Diameter (GID) (mm) | Fluconazole GID (mm) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 μL | 10 μL | 15 μL | 150 mg | |||||||||||||||
| C. albicans | 7 | 8 | 10 | 24.7 | ||||||||||||||
| Candida albicans | 32 | 32 | 1 | C. tropicalis | 9 | 11 | 13 | |||||||||||
| Candida tropicalis | 25 | |||||||||||||||||
| 64 | 64 | 1 | C. glabrata | 9 | 11 | 11 | 23.7 | |||||||||||
| Candida glabrata | 32 | 32 | 1 | C. dubliniensis | 8 | 10 | 11 | 19.3 | ||||||||||
| Candida dubliniensis | 32 | 32 | 1 | Candida | sp. | 7 | 8 | 10 | 24 | |||||||||
| Candida | sp. | 16 | 6 | trans | -Pinocarveol | C | 10 | H | 16 | O | 11.02 | 1110 | 1139 | |||||
| R. rubra | 8 | 10 | 12 | 18.7 | ||||||||||||||
| 62 | 4 | |||||||||||||||||
| Rhodotorula rubra | 32 | 32 | 1 | 7 | Pinocarvone | C | 10 | H | 14 | O | 2.72 | 1125 | 1164 | |||||
| A. niger | - | 7 | 8 | 13.7 | ||||||||||||||
| Cryptoccocus neoformans | 64 | 64 | 1 | 8 | C. neoformans | Borneol | C | 10 | H | 18 | O | 4.80 | 1137 | 1169 | ||||
| 9 | 11 | 13 | 29.3 | 9 | Terpinen-4-ol | |||||||||||||
| Penicillium | sp. | C | 10 | H | 18 | O | 4.09 | 1151 | 1177 | |||||||||
| 7 | 10 | Myrtenal | C | 10 | H | 14 | O | 1.76 | 1156 | 1195 | ||||||||
| 10 | 11 | Myrtenol | C | 10 | H | 16 | O | ( | Z | )-β-Damascone | C | 13 | H | 20 | O | 0.97 | 1238 | 1387 |
| 15 | Bornylacetate | C | 12 | H | 20 | O | 2 | 9.38 | 1259 | 1285 | ||||||||
| MEM 10 μg | 16 | Carvacrol | C | 10 | H | 14 | O | 0.80 | 1274 | 1084 | ||||||||
| R | TET 30 μg | S | 4.02 | 1168 | 1195 | |||||||||||||
| 12 | trans- | Carveol | C | 10 | H | 17 | Myrtenylacetate | C | 12 | H | 18 | O | 2 | 1.00 | 1296 | 1326 | ||
| 18 | 2,4,6-trimethoxytoluene | C | 10 | H | 14 | O | 3 | 0.61 | 1298 | 1483 | ||||||||
| 19 | ( | E | )-Trimenal | C | 13 | H | 22 | O | 0.77 | 1343 | 1421 | |||||||
| 20 | Aromadendrene | C | 15 | H | 24 | 0.67 | 1438 | 1441 | ||||||||||
| 21 | Viridiflorene | C | 15 | H | 24 | 0.95 | 1473 | 1496 | ||||||||||
| 22 | 2,3-Dihydro-1,1,4,5,6-pentamethyl 1 | H | -indene | C | 14 | H | 20 | 0.81 | 1481 | 1522 | ||||||||
| 23 | cis | -Calamenene | C | 15 | H | 22 | 1.17 | 1493 | 1529 | |||||||||
| 24 | δ-Cadinene | C | 15 | H | 24 | 0.68 | 1500 | 1523 | ||||||||||
| 25 | Palustrol | C | 15 | H | 26 | O | 1.14 | 1538 | 1568 | |||||||||
| 26 | Spathulenol | C | 15 | H | 24 | O | 1.41 | 1542 | 1578 | |||||||||
| 27 | Caryophyllene oxide | C | 15 | H | 24 | O | 2.85 | 1640 | ||||||||||
| 32 | Cadalene | |||||||||||||||||
| TIM 85 μg | R | CEF 15 μg | S | |||||||||||||||
| ATB | E. coli | ATB | S. Typhi | |||||||||||||||
| COL 50 μg | S | 1546 | 1583 | |||||||||||||||
| COL 50 μg | S | |||||||||||||||||
| MEM 10 μg | S | MEM 10 μg | S | |||||||||||||||
| TIC 75 μg | R | TIC 75 μg | S | |||||||||||||||
| AMI 30 μg | S | AMI 30 μg | S | 12 | 13.8 | |||||||||||||
| Fusarium | sp. | - | 7 | 8 | 16.3 | |||||||||||||
Minimum Inhibitory Concentrations (MIC) and Minimum Fungicide Concentrations (MFC)
Table 7 summarizes the values of the MIC and MFC of the essential oil determined in a liquid medium against the ten tested fungal strains. The essential oil of C. ladanifer showed an antifungal activity on all fungal strains tested with concentrations ranging between 16 and 64 μL/mL.
Table 7. The effect of the C. ladanifer essential oil on the MIC and MFC of the selected fungi strains.
| Fungal Strains | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MFC/MIC | |||||||||
| Penicillium | |||||||||||
| sp. | |||||||||||
| 64 | |||||||||||
| 64 | |||||||||||
| 1 | |||||||||||
| Fusarium | sp. | 64 | 64 | 1 | |||||||
| Aspergillus niger | 32 | 32 | |||||||||
| 16 | |||||||||||
| O | 1.44 | 1189 | 1216 | ||||||||
| 13 | Carvone | C | 10 | H | 14 | O | 0.54 | 1203 | 1243 | ||
| 14 | |||||||||||
| 28 | |||||||||||
| Viridiflorol | |||||||||||
| C | |||||||||||
| 15 | |||||||||||
| H | |||||||||||
| 26 | |||||||||||
| O | |||||||||||
| 17.74 | |||||||||||
| 1560 | 1592 | ||||||||||
| 1 | 29 | Ledol | C | 15 | H | 26 | O | 8.85 | 1570 | 1602 | |
| 30 | 1,10-di- | epi | -Cubenol | C | 15 | H | 26 | O | 0.75 | 1595 | 1619 |
| 31 | Caryophylla-4 (12), 8 (13)-dien-5α-ol | C | 15 | H | 24 | ||||||
| C | 15 | H | 18 | 0.82 | 1634 | 1676 | |||||
| 33 | 14-Hydroxy-4,5-dihydro- caryophyllene |
C | 15 | H | 26 | O | 0.64 | 1848 | 1706 | ||
| 34 | Sclareol | C | 20 | H | 36 | O | 2 | 4.60 | 1924 | 2223 | |
| 35 | 13- | epi | -Dolabradiene | C | 20 | H | 32 | 1.28 | 2013 | 2000 | |
| Oxygenated sesquiterpenes | 34.02 | ||||||||||
| Oxygenated monoterpenes | 33.14 | ||||||||||
| Linear esters | 10.38 | ||||||||||
| Monoterpenes | 9.11 | ||||||||||
| Sesquiterpenes | 4.29 | ||||||||||
| Others | 9.06 | ||||||||||
| Total | 100 | ||||||||||
2.5. Antibacterial Activity
In this study, the antibacterial activity was evaluated using two methods: the agar disk diffusion method and the dilution in a liquid environment. The aim of the tests is to highlight the inhibitory power of the essential oil vis-à-vis the tested bacteria after 24 h of incubation at an adequate temperature of 37 °C.
S: Sensitive; I: Intermediate; A: Resistant; ATB: Antibiotics; TIC: Ticarcillin; CEF: Ceftazidime; VAN: Vancomycin; TET: Tetracycline; CEF: Cefalexin; COL: Colistin; AMI: Amikacin.
2.5.2. The Agar Disk-Diffusion Method for C. ladanifer Essential Oil
Table 4 and Figure 2 represent the results of the agar disk-diffusion method for C. ladanifer essential oil against the selected bacterial strains after 24 h at 37 °C. The agar disk diffusion method results indicated that the essential oil of C. ladanifer has a remarkable antibacterial activity compared to the concentration used (5 μL). From the analysis of the results obtained (Table 4), it was noted that the four microorganisms studied are sensitive except S. Typhi which demonstrated an inhibition zone of 30 ± 0.25 mm when the EO of C. ladanifer was used. The best inhibition diameter was against S. aureus (55 ± 0.22 mm) and E. coli (42 ± 0.11). It was also noted that contrary to the sensitivity test when A. baumannii
The results of the MFC were all compared to those of MIC except for Candida sp. strain. Those obtained results were interesting and in agreement with those obtained by Guinoiseau et al., Rossi et al., Vieira et al. which indicate that the essential oil from C. ladanifer has an antimicrobial activity on fungi (such as Aspergillus niger, Botrytis cinerea, Mucorracemosus, and Verticilliumalboatrum) [38,39,40][38][39][40].
3. Discussions on Essential Oil from Cistus ladanifer L.
This study, among others, focused on revealing the potential bioactivity of the essential oil of C. ladanifer. The study’s novelty was that plant material was collected during its time of flowering and in a different region (Middle Atlas), which is known for its semi-arid climate. The edaphic and climate parameters and others have influenced the variation of the composition revealed in the chromatographic analysis. The essential oil major components were viridiflorol (17.64%), trans-pinocarveol (11.02%), bornyl acetate (9.38%), and ledol (8.85%) indicating the domination of the oxygenated sesquiterpenes (34.02%), oxygenated monoterpenes (33.14%) in the overall composition.
The oxygenated sesquiterpenes and monoterpenes are a well-known group of compounds with antibacterial and antifungal proprieties [41,42][41][42] and their presence among the EO composition explains the majority of the outstanding outcomes obtained.
The strains chosen for this research are of great interest in the areas of clinical and public health. Their increasing resistance to conventional drugs has prompted further research into new, more effective options, particularly natural products [43,44][43][44]. S. aureus (Gram-positive) is a member of the indigenous human microflora and may be found asymptomatically in a variety of bodily locations. Diseases caused by transmission from these locations are both endemic and epidemic [45]. Infection with S. aureus is a leading cause of skin, soft tissue, respiratory, bone, joint, and endovascular diseases. Many strains of S. aureus are becoming resistant to current antibacterial treatments, posing a significant issue in medical microbiology [46].
S. Typhi, one of the representatives of the Salmonella family, is the direct causative organism of typhoid fever [47] (accompanied by weakness, headaches, mild vomiting, abdominal pain, and constipation). Symptoms may persist for weeks or months if not treated [48].
E. coli usually colonizes human babies’ gastrointestinal tracts within a few hours after birth. E. coli and its human host often live in excellent health and mutual benefit for decades [48]. These commensal E. coli strains seldom cause illness unless the host is immunocompromised or the usual gastrointestinal barriers are broken, as in peritonitis. Diarrhea induced by E. coli infection is a growing issue in both the developing and developed worlds, with significant rates of death in newborn infants and animals [49]. Although most commensal representatives found in human and animal gut flora are non-pathogenic, certain strains are very dangerous.
The genus Acinetobacter, over the past 30 years, has experienced considerable taxonomic evolution. Its most prominent example, A. baumannii, has emerged as one of the most problematic infections for healthcare facilities worldwide [50]. A. baumannii strains resistant to all known antibiotics have now been discovered, indicating a sentinel occurrence that should be addressed by the worldwide health care community as soon as possible. It often attacks the most susceptible hospitalized patients, those who are severely sick and have compromised skin integrity and airway protection [51].
In terms of fungus, non-albicans candida species are increasingly being reported as both colonizers and pathogens causing nosocomial fungal bloodstream infections, accounting for nearly half of all non-superficial candida infections, with C. glabrata, C. tropicalis, and C. dubliniensis being the most common [52].
One other life-threatening fungi exploited in this study is C. neoformans which is responsible for cryptococcal meningitis, the most prevalent type of cryptococcosis, often chronic and deadly if left untreated [53]. This virulence is none less than those presented by other fungi such as Aspergillus niger, a fungus that causes the “black mold” on certain fruits and vegetables (contaminant of food) which its consumption (as it secretes ochratoxins –mycotoxins) causes nephrotoxicity and renal tumors [54]. The EO of C. ladanifer and through this study demonstrated a strong and real potential that could be better exploited to fight against the threats presented by all the microbial strains studied with slight differences in terms of efficacy.
References
- Es-safi, I.; Mechchate, H.; Amaghnouje, A.; Elbouzidi, A.; Bouhrim, M.; Bencheikh, N.; Hano, C.; Bousta, D. Assessment of Antidepressant-Like, Anxiolytic Effects and Impact on Memory of Pimpinella anisum L. Total Extract on Swiss Albino Mice. Plants 2021, 10, 1573.
- Bencheikh, N.; Elbouzidi, A.; Kharchoufa, L.; Ouassou, H.; Alami Merrouni, I.; Mechchate, H.; Es-safi, I.; Hano, C.; Addi, M.; Bouhrim, M.; et al. Inventory of Medicinal Plants Used Traditionally to Manage Kidney Diseases in North-Eastern Morocco: Ethnobotanical Fieldwork and Pharmacological Evidence. Plants 2021, 10, 1966.
- Herrera, C.M. Tipos Morfológicos y Funcionales En Plantas Del Matorral Mediterráneo Del Sur de España. Stud. Oecologica 1984, 5, 7–34.
- Nuñez, E. Ecología Del Jaral de Cistus ladanifer L. Ph.D. Thesis, Facultad de Ciencias, Universidad de Extremadura, Badajoz, Spain, 1989.
- Mariotti, J.P.; Tomi, F.; Casanova, J.; Costa, J.; Bernardini, A.F. Composition of the Essential Oil of Cistus ladaniferus L. Cultivated in Corsica (France). Flavour Fragr. J. 1997, 12, 147–151.
- Bellakhdar, J. Medecine arabe ancienne et savoirs populaires. In La Pharmacopée Traditionnelle; Ibis Press: Paris, France, 1997.
- Talavera, S.; Gibbs, P.E.; Herrera, J. Reproductive Biology of Cistus ladanifer (Cistaceae). Plant Syst. Evol. 1993, 186, 123–134.
- Devesa Alcaraz, J.A. Vegetación y Flora de Extremadura; Universitas Editorial: Badajoz, Spain, 1995.
- Pomponio, R.; Gotti, R.; Santagati, N.A.; Cavrini, V. Analysis of Catechins in Extracts of Cistus Species by Microemulsion Electrokinetic Chromatography. J. Chromatogr. A 2003, 990, 215–223.
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Saura, D.; Guillén, E.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae Aqueous Extracts Containing Ellagitannins Show Antioxidant and Antimicrobial Capacity, and Cytotoxic Activity against Human Cancer Cells. Food Chem. Toxicol. 2010, 48, 2273–2282.
- Andrade, D.; Gil, C.; Breitenfeld, L.; Domingues, F.; Duarte, A.P. Bioactive Extracts from Cistus ladanifer and Arbutus unedo L. Ind. Crops Prod. 2009, 30, 165–167.
- Benayad, N.; Mennane, Z.; Charof, R.; Hakiki, A.; Mosaddak, M. Antibacterial Activity of Essential Oil and Some Extracts of Cistus ladaniferus from Oulmes in Morocco. J. Mater. Environ. Sci. 2013, 4, 1066–1071.
- Bonnier, G.; Douin, R.; Poinsot, J.; Palese, R.; Aeschimann, D. La Grande Flore en Couleurs de Gaston Bonnier: France, Suisse, Belgique et Pays Voisins; P. Parey: Paris, France; Hamburg, Germany; Berlin, Germany, 1990; ISBN 978-2-7011-1300-5.
- Di Pasqua, R.; De Feo, V.; Villani, F.; Mauriello, G. In Vitro Antimicrobial Activity of Essential Oils from Mediterranean Apiaceae, Verbenaceae and Lamiaceae against Foodborne Pathogens and Spoilage Bacteria. Ann. Microbiol. 2005, 55, 139–143.
- Oller-López, J.L.; Rodríguez, R.; Cuerva, J.M.; Oltra, J.E.; Bazdi, B.; Dahdouh, A.; Lamarti, A.; Mansour, A.I. Composition of the Essential Oils of Cistus ladaniferus and C. monspeliensis from Morocco. J. Essent. Oil Res. 2005, 17, 553–555.
- Youbi, A.; El Mansouri, L.; Boukhira, S.; Daoudi, A.; Bousta, D. In Vivo Anti-Inflammatory and Analgesic Effects of Aqueous Extract of Cistus ladanifer L. Moroc. Am. J. Ther. 2016, 23, e1554–e1559.
- Mekhfi, H.; El Haouari, M.; Legssyer, A.; Bnouham, M.; Aziz, M.; Atmani, F.; Remmal, A.; Ziyyat, A. Platelet Anti-Aggregant Property of Some Moroccan Medicinal Plants. J. Ethnopharmacol. 2004, 94, 317–322.
- Guimarães, R.; Sousa, M.J.; Ferreira, I.C.F.R. Contribution of Essential Oils and Phenolics to the Antioxidant Properties of Aromatic Plants. Ind. Crops Prod. 2010, 32, 152–156.
- Mohammed, B.; Said, C.; Fouzia, F.R.; Kawtar, F.B.; Zoubida, H.; Abdelilah, O.; Mohammed, E.; Ghizlane, E. Chemical Composition and Antimicrobial Activity of the Essential Oil of Cistus ladanifer Var. Maculatus Dun. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 925–930.
- Aziz, M.; Tab, N.; Karim, A.; Mekhfi, H.; Bnouham, M.; Ziyyat, A.; Melhaoui, A.; Legssyer, A. Relaxant Effect of Aqueous Extract of Cistus ladaniferus on Rodent Intestinal Contractions. Fitoterapia 2006, 77, 425–428.
- Attaguile, G.; Perticone, G.; Mania, G.; Savoca, F.; Pennisi, G.; Salomone, S. Cistus incanus and Cistus monspeliensis Inhibit the Contractile Response in Isolated Rat Smooth Muscle. J. Ethnopharmacol. 2004, 92, 245–250.
- Skorić, M.; Todorović, S.; Gligorijević, N.; Janković, R.; Živković, S.; Ristić, M.; Radulović, S. Cytotoxic Activity of Ethanol Extracts of in Vitro Grown Cistus creticus Subsp. creticus L. on Human Cancer Cell Lines. Ind. Crops Prod. 2012, 38, 153–159.
- Sosa, T.; Chaves, N.; Alias, J.C.; Escudero, J.C.; Henao, F.; Gutiérrez-Merino, C. Inhibition of Mouth Skeletal Muscle Relaxation by Flavonoids of Cistus ladanifer L.: A Plant Defense Mechanism against Herbivores. J. Chem. Ecol. 2004, 30, 1087–1101.
- Belmokhtar, M.; Bouanani, N.E.; Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Aziz, M.; Matéo, P.; Fischmeister, R.; Legssyer, A. Antihypertensive and Endothelium-Dependent Vasodilator Effects of Aqueous Extract of Cistus Ladaniferus. Biochem. Biophys. Res. Commun. 2009, 389, 145–149.
- Amensour, M.; Sendra, E.; Pérez-Alvarez, J.A.; Skali-Senhaji, N.; Abrini, J.; Fernández-López, J. Antioxidant Activity and Chemical Content of Methanol and Ethanol Extracts from Leaves of Rockrose (Cistus ladaniferus). Plant Foods Hum. Nutr. 2010, 65, 170–178.
- Lahcen, S.A.; El Hattabi, L.; Benkaddour, R.; Chahboun, N.; Ghanmi, M.; Satrani, B.; Tabyaoui, M.; Zarrouk, A. Chemical Composition, Antioxidant, Antimicrobial and Antifungal Activity of Moroccan Cistus creticus Leaves. Chem. Data Collect. 2020, 26, 100346.
- Robles, C.; Bousquet-Mélou, A.; Garzino, S.; Bonin, G. Comparison of Essential Oil Composition of Two Varieties of Cistus ladanifer. Biochem. Syst. Ecol. 2003, 31, 339–343.
- Bechlaghem, K.; Allali, H.; Benmehdi, H.; Aissaoui, N.; Flamini, G. Chemical Analysis of the Essential Oils of Three Cistus Species Growing in North-West of Algeria. Agric. Conspec. Sci. 2019, 84, 283–293.
- Zidane, H.; Elmiz, M.; Aouinti, F.; Tahani, A.; Wathelet, J.; Sindic, M.; Elbachiri, A. Chemical Composition and Antioxidant Activity of Essential Oil, Various Organic Extracts of Cistus ladanifer and Cistus libanotis Growing in Eastern Morocco. Afr. J. Biotechnol. 2013, 12, 5314–5320.
- Greche, H.; Mrabet, N.; Zrira, S.; Ismaili-Alaoui, M.; Benjilali, B.; Boukir, A. The Volatiles of the Leaf Oil of Cistus ladanifer L. Var. Albiflorus and Labdanum Extracts of Moroccan Origin and Their Antimicrobial Activities. J. Essent. Oil Res. 2009, 21, 166–173.
- Hellal, Z. Contribution à l’étude Des Propriétés Antibactériennes et Antioxydantes de Certaines Huiles Essentielles Extraites Des Citrus. Application Sur La Sardine (Sardina pilchardus). Doctoral Dissertation, Université Mouloud Mammeri, Tizi Ouzou, Algeria, 2011.
- Mrabkt, N.; Lahlou, H.; Benjilali, B. Effet de Quelques Extraits Du Ciste Ladanifère Du Maroc (Cistus ladaniferus L.) Sur La Croissance de Quatre Champignons. Cryptogam. Mycol. 1999, 20, 23–33.
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Chemical Composition and Herbicidal Activity of the Essential Oil from a Cistus ladanifer L. Population from Spain. Nat. Prod. Res. 2012, 26, 1602–1609.
- Gomes, P.B.; Mata, V.G.; Rodrigues, A.E. Characterization of the Portuguese-Grown Cistus Ladanifer Essential Oil. J. Essent. Oil Res. 2005, 17, 160–165.
- Sadeq, O.; Mechchate, H.; Es-safi, I.; Bouhrim, M.; Zahra Jawhari, F.; Ouassou, H.; Kharchoufa, L.; AlZain, M.N.; Alzamel, N.M.; Mohamed Al kamaly, O.; et al. Phytochemical Screening, Antioxidant and Antibacterial Activities of Pollen Extracts from Micromeria Fruticosa, Achillea Fragrantissima, and Phoenix Dactylifera. Plants 2021, 10, 676.
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The Effectiveness of Plant Essential Oils on the Growth of Botrytis cinerea, Fusarium sp. and Clavibacter Michiganensis subsp. Michiganensis. Crop Prot. 2003, 22, 39–44.
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571.
- Guinoiseau, E.; Lorenzi, V.; Luciani, A.; Tomi, F.; Casanova, J.; Berti, L. Susceptibility of the Multi-Drug Resistant Strain of Enterobacter Aerogenes EA289 to the Terpene Alcohols from Cistus ladaniferus Essential Oil. Nat. Prod. Commun. 2011, 6, 1159–1162.
- Rossi, P.-G.; Berti, L.; Panighi, J.; Luciani, A.; Maury, J.; Muselli, A.; de Rocca Serra, D.; Gonny, M.; Bolla, J.-M. Antibacterial Action of Essential Oils from Corsica. J. Essent. Oil Res. 2007, 19, 176–182.
- Vieira, M.; Bessa, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.S.; Mendes, Â.; Martins da Costa, P.; Belo, A.D.F. Chemical Composition, Antibacterial, Antibiofilm and Synergistic Properties of Essential Oils from Eucalyptus globulus Labill. and Seven Mediterranean Aromatic Plants. Chem. Biodivers. 2017, 14, e1700006.
- da Costa, J.S.; de Figueiredo, R.O.; Setzer, W.N.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties. Molecules 2021, 26, 965.
- Meng, J.C.; Zhu, Q.X.; Tan, R.X. New Antimicrobial Mono-and Sesquiterpenes from Soroseris hookeriana subsp. erysimoides. Planta Med. 2000, 66, 541–544.
- Mechchate, H.; Costa de Oliveira, R.; Es-safi, I.; Vasconcelos Mourão, E.M.; Bouhrim, M.; Kyrylchuk, A.; Soares Pontes, G.; Bousta, D.; Grafov, A. Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds. Pharmaceuticals 2021, 14, 770.
- Bouhrim, M.; Ouassou, H.; Boutahiri, S.; Daoudi, N.E.; Mechchate, H.; Gressier, B.; Eto, B.; Imtara, H.; Alotaibi, A.A.; Al-zharani, M.; et al. Opuntia dillenii (Ker Gawl.) Haw., Seeds Oil Antidiabetic Potential Using In Vivo, In Vitro, In Situ, and Ex Vivo Approaches to Reveal Its Underlying Mechanism of Action. Molecules 2021, 26, 1677.
- Fa, W. Staphylococcus Aureus (Including Toxic Shock Syndrome). In Principles and Practices of Infectious Diseases, 4th ed.; Churchill Livingstone Elsevier: Philadelphia, PA, USA, 1995; Volume 4, pp. 1754–1783.
- Chambers, H.F. Methicillin Resistance in Staphylococci: Molecular and Biochemical Basis and Clinical Implications. Clin. Microbiol. Rev. 1997, 10, 781–791.
- Arshad, R.; Pal, K.; Sabir, F.; Rahdar, A.; Bilal, M.; Shahnaz, G.; Kyzas, G.Z. A Review of the Nanomaterials Use for the Diagnosis and Therapy of Salmonella Typhi. J. Mol. Struct. 2021, 1230, 129928.
- da Cruz, L.F.; Souza, I.L.A.; de Souza, L.D.; de Freitas Araújo, M.G.; Granjeiro, P.A. The Importance of Intestinal Microbiota and Its Role in the Nosocomial Infection. Res. Soc. Dev. 2021, 10, e489101019166.
- Radu, S.; Ling, O.W.; Rusul, G.; Karim, M.I.A.; Nishibuchi, M. Detection of Escherichia coli O157: H7 by Multiplex PCR and Their Characterization by Plasmid Profiling, Antimicrobial Resistance, RAPD and PFGE Analyses. J. Microbiol. Methods 2001, 46, 131–139.
- Fournier, P.E.; Richet, H.; Weinstein, R.A. The Epidemiology and Control of Acinetobacter Baumannii in Health Care Facilities. Clin. Infect. Dis. 2006, 42, 692–699.
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer Membrane Protein A (OmpA) as a Potential Therapeutic Target for Acinetobacter Baumannii Infection. J. Biomed. Sci. 2020, 27, 1–8.
- Ann Chai, L.Y.; Denning, D.W.; Warn, P. Candida Tropicalis in Human Disease. Crit. Rev. Microbiol. 2010, 36, 282–298.
- Zhao, Y.; Lin, J.; Fan, Y.; Lin, X. Life Cycle of Cryptococcus Neoformans. Annu. Rev. Microbiol. 2019, 73, 17–42.
- Cairns, T.C.; Nai, C.; Meyer, V. How a Fungus Shapes Biotechnology: 100 Years of Aspergillus Niger Research. Fungal Biol. Biotechnol. 2018, 5, 1–14.
More
