Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Pingfang Yang.
Flower color is one of the most prominent traits of rose flowers and determines their ornamental value. The ‘Chen Xi’ variety of rose has a very beautiful flower showing color changes during the blooming, which contributes a lot to its ornamental value.
- rose flower
- alteration color
- flavonoid metabolites
- anthocyanins
- light induction
1. Introduction
The rose (Rosa sp.), belonging to the Rosaceae family, is one of the most popular and widely planted ornamental plants worldwide. It is also one of the top ten flowers in China, which is honored as the queen of flowers and has a long history of cultivation in China [1]. Roses are utilized as cut flowers, potted plants, and garden ornamental plants [2,3][2][3]. Due to their rich bioactive components, rose flowers are extensively used in food, drugs, cosmetics, and pharmaceutics [4,5,6,7][4][5][6][7]. Rose flowers are charming and colorful, including red, pink, orange, yellow, white, variegated, as well as alternating colors during flowering. Additionally, the blue rose flower has been cultivated through the molecular breeding method [8]. The color of the rose flower determines its ornamental and commercial value and creating novel flower colors is one of the main objectives for breeders.
It is known to all that plant color is determined by pigments, and there are four kinds of natural pigments that include flavonoids, carotenoids, chlorophyll, and alkaloids [9], which give color to plant flowers, leaves, vegetables, and fruits. Osterc [9] detected several phenolic compounds from rose petals of eight cultivars at four developmental stages, including five anthocyanins, which were regarded as the major factor for visual attributes of rose flower, and suggested that the total content of anthocyanin and quercetin (both belonging to flavonoids) continued accumulating from the bud stage until fully open flowers, then decreased in senescent ones. Previous studies indicated that in rose flowers, anthocyanins, determine the red color, carotenoids impart yellow color, while pink and orange colors are endowed by a combination of both anthocyanins and carotenoids, and nearly no pigments were detected in white flowers [10]. Lee et al. [11] identified two anthocyanins, cyanidin 3,5-di-O-glucoside and pelargonidin 3,5-di-O-glucoside in red rose flowers, with the former occupying 85% of total anthocyanins. Wan et al. [3] found nineteen flavonols and sixteen carotenoids in yellow petals of Rosa ‘Sun City’ cultivar, then they also detected four anthocyanins, 20 flavonols, and 10 carotenoids in the petals of six Rosa cultivars with yellow, pink, and orange color [10]. Combined with RNA-sequencing and metabolite analysis, Huang et al. [12] revealed the mechanism of flower color change in rose mutants. The results indicated that the expression levels of the differentially expressed genes enriched in the anthocyanin pathway, e.g., chalcone synthase (CHS), chalcone isomerase (CHI), dihydroflavonol reductase (DFR), and leucoanthocyanidin dioxygenase (LDOX) in pink flowers were significantly higher than in white flowers, which are consistent with the accumulation of anthocyanin in flowers of the two rose cultivars. By contrast, the expression of flavonol synthase (FLS) in the white rose flower was higher than that in red flowers, resulting in the accumulation of more flavonols. Therefore, these findings suggested that competition between anthocyanin and flavonol biosynthesis is a primary cause of rose flower color variation [12].
Light affects anthocyanin biosynthesis: for example, the anthocyanin pigmentation of some fruits’ skin including red pears [13], apples [14], and grapes [15] is induced by light. In this study, we chose one rose cultivar whose flower color changed from yellow to red during development. Before the flower opens and the petal emerges from the calyx, the flower petals are yellow with an edge which, when exposed to the sun, become red, and after the flower totally opens and the petals are fully stretched, the red color intensifies with increased exposure to the sunlight. As the flower ages, the color of the petals slowly faded to white and wither at the end. However, when the flower fully opens and is moved to the shade, the color of the yellow petals persists and does not change to red and directly fades to white as it withers. To detect if the color change is induced by light, flavonoid metabolites of rose flowers at four developmental stages under natural conditions as well as one stage flower under shading treatment were determined. The results showed the dynamic change of flavonoid metabolites during the process of color change with the flower development and lay a theoretical foundation for further understanding of the mechanism underlying the light-induced rose flower pigmentation and ultimately facilitate breeding of rose cultivars with a novel flower color.

2. Light-Induced Color Changes of Rose Petals
The ‘Chen Xi’ variety of rose has a very beautiful flower showing color changes during the blooming, which contributes a lot to its ornamental value. At the bud stage, sepals show a little red color in the green background and cover the whole flower (Figure 1A). After the sepals opened, petals on the surface showed some red color with a yellow background. When the flower was just fully opened, the petals were in yellow with a tiny amount of red at the edge. Along with its development and blooming, the red is gradually increased, and then both red and yellow were gradually faded with the wilting of petals (Figure 1A). Based on the changing style of the petal’s color, we suspected that the formation of the red color is light-inducible. To verify this hypothesis, two different light treatments were carried out on the newly bloomed flowers in May 2020, natural sunlight (control group) and shading by being wrapped in a black opaque paper bag (Figure 1B). Rose flower petals at four developmental stages (named as S1, S2, S3 and S4) were observed to compare the difference of color changes between the two light treatments. At the S1 stage corresponding to the newly opened flower (Figure 1(A5)), flowers under both treatments were mainly yellow; at the S2 stage, the flower is mainly yellow with some light red (Figure 1(A7),B) under natural light, while it stayed as yellow under darkness (Figure 1); at the S3 stage, the color was deep red with fading yellow under light (Figure 1(A9),B), and flowers in the dark showed only fading yellow; at the S4 stage, the yellow was totally faded under both treatments, while only the flowers with light still showed red, although it was fading as well (Figure 1(A11),B). Altogether, it could be concluded that the changes in the red color are light dependent, but the accumulation and fading of yellow is not.
Figure 1. Changes in flower colors of the variety of ‘Chen Xi’ rose. (A) The different developmental stages of ‘Chen Xi’ rose flower from bud stage to flower blooming, and the flower color changed from yellow to red, and faded to white as it withers ((1–12) represent the order of the development stages of ‘Chen Xi’ flower). (B) ‘Chen Xi’ flowers at four blooming stages under natural sunlight (S1–S4) and shading (S1D–S4D).
3. Metabolic Profiling of Rose Petals at Different Development Stages
To explore the mechanism underlying this light-induced color change, the flavonoid metabolites of the rose petals from four different development stages and petals at the S2D stage under shading treatment (Figure 1B) were investigated by UPLC-ESI-MS/MS. A total of 176 flavonoid components were detected, including 49 flavones, 59 flavonols, 12 flavanones, 3 isoflavones, 12 anthocyanins, and 41 proanthocyanidins (Table S1), and HPLC chromatograms of these flavonoid metabolites from rose petal samples were shown in Figure S1. Principal components analysis (PCA) was carried out to assess the repeatability among three biological repeats and the variation among different samples. In the present study, two principal components PC1 and PC2 were extracted, which contributed 33.6% and 16.9%, respectively. The mix represents a sample for quality control, and PCA shows separation of the different samples and good repeatability (Figure 2). Additionally, samples of S2D could be separated from other samples by PCA (Figure 2).
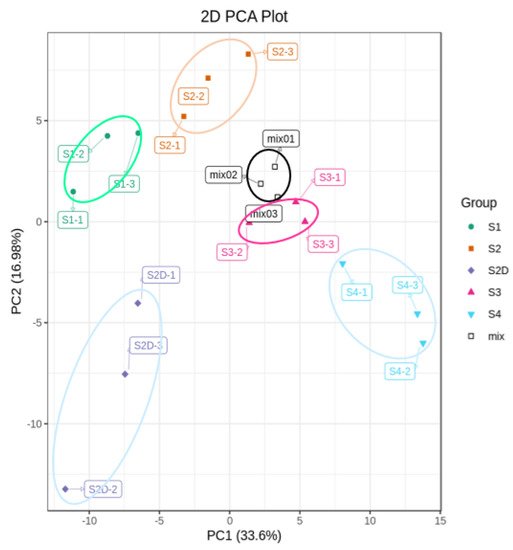

Figure 2. Principal component analysis (PCA) of flavonoid metabolites profiles analysis.
All the metabolites are illustrated by a heatmap, and six groups were classified based on the content of metabolites in different samples (Figure 3A). Metabolites in Group I, which had the highest number of compounds (52), mainly composed of flavones, flavonols and proanthocyanidins, had lower content in the petals of S1, then most of them increased along with the growth of the rose flower development, and there was no significant difference between S1 and S2D petals, but most of the metabolites were higher in S2 than S2D petals, while most metabolites in group II, including flavones, proanthocyanidins, flavanols, and flavonols, had the lowest content in the shading treatment (in S2D petals) but had the highest content in S2 petals. In group III, some metabolites exhibited no difference between S1 and S2 petals, then slowly increased in S3 and accumulated more in S4 petals, while some were lower in S1 petals, increased in S2 and S3, then sharply down regulated in S4 petals. Most of the metabolites in this group had higher content in petals S2D than that in S1 and S2 petals. This group also mainly contained flavones, flavonols and proanthocyanidins. Group IV had the lowest number of members, including four flavones, four flavonols, one flavanol, one anthocyanin and one proanthocyanin, almost all of them showed the highest content in S1 petals, and decreased rapidly in S2, and kept declining in S3 and S4 petals, but only several members exhibited up-regulation in S4. The majority of metabolites in the S2D of this group had slightly lower content than those in S1. In group V, flavonols and proanthocyanidins were the main members, and they kept a high content in the first two stages petals but showed a continued descent in petals of S3 and S4 petals, with most metabolites being down-regulated in S2D petals. Unlike other groups, most of the flavonoid metabolites in group VI, which included 12 flavones, 8 proanthocyanidins, 4 flavonols, 2 anthocyanins, and 1 flavanol, had the highest content in S2D, while they had a lower expression in S1, S3, and S4 flowers (Figure 3A). Specifically, eriodictyol (dihydroflavone), quercetin-3-O-(2″-acetyl)-glucosylgalactoside (flavonol), cyanidin-3-O-glucoside (kuromanin), and cyanidin-3-O-(6″-malonylglucoside) (two anthocyanins) showed a dramatically light-induced pattern, whereas two proanthocyanidins valoneoyl-glucose and procyanidin A6 showed an opposite pattern (Table 1).
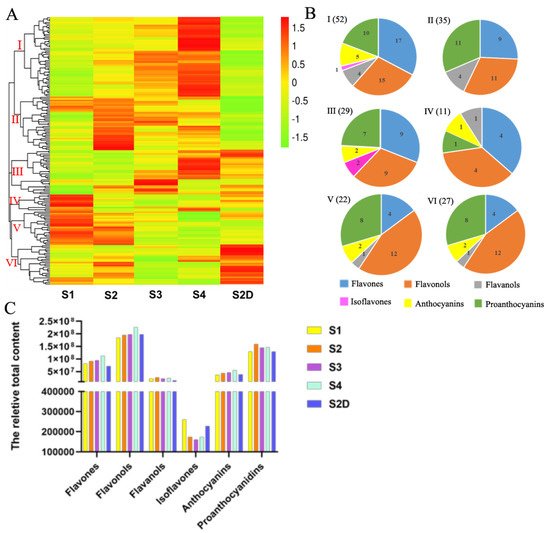

Figure 3. Flavonoid metabolites profile analysis. (A) Clustering heat map of all flavonoid metabolites, and (B) pie charts of the metabolites in each group. (C) The relative content of six types of flavonoid compounds, the content value was calculated by the peak area of each metabolite.
Table 1. The information of differential metabolites from five comparison groups.
| Index | MW (Da) | Ionization Model | Compounds | Class | S2/S1 | S3/S2 | S4/S3 | S2D/S1 | S2D/S2 |
|---|---|---|---|---|---|---|---|---|---|
| pme2960 | 272.07 | [M+H]+ | Naringenin chalcone * | Chalcones | 0.72 / | 0.29 | 0.70 / | 0.38 | 0.52 / |
| pme1201 | 274.08 | [M−H]− | Phloretin | Chalcones | 3.29 | 0.31 | 1.44 / | 0.93 / | 0.28 |
| pme0376 | 272.07 | [M−H]− | Naringenin | Dihydroflavone | 0.69 / | 0.29 | 0.78 / | 0.36 | 0.52 / |
| Hmqp004476 | 272.07 | [M+H]+ | 5,7,4′-Trihydroxydihydroflavone * | Dihydroflavone | 1.15 / | 0.32 | 1.06 / | 0.66 / | 0.57 / |
| mws0064 | 288.06 | [M−H]− | Eriodictyol | Dihydroflavone | 19.10 | 0.05 | 1.75 / | 1.29 / | 0.07 |
| mws1094 | 288.06 | [M−H − | Dihydrokaempferol | Dihydroflavonol | 0.50 | 0.09 | 0.42 | 0.46 | 0.92 / |
| mws0044 | 304.06 | [M−H]− | Dihydroquercetin (Taxifolin) | Dihydroflavonol | 1.31 / | 0.60 / | 0.63 / | 0.61 / | 0.47 |
| mws1174 | 314.08 | [M−H]− | 3-O-Acetylpinobanksin | Dihydroflavonol | 7.90 | 1.06 / | 3.98 | 2.84 | 0.36 |
| mws0040 | 254.06 | [M+H]+ | Chrysin | Flavones | 6.42 | 1.35 / | 3.33 | 2.11 | 0.33 |
| mws0051 | 284.07 | [M+H]+ | Acacetin | Flavones | 2.57 | 1.49 / | 0.55 / | 2.15 | 0.84 / |
| mws0058 | 300.06 | [M−H]− | Diosmetin | Flavones | 3.16 | 0.83 / | 0.51 / | 2.82 | 0.89 / |
| Zmhp004065 | 344.09 | [M+H]+ | 7,8-Dihydroxy-5,6,4′-trimethoxyflavone * | Flavones | 6.58 | 0.99 / | 1.79 / | 0.87 / | 0.13 |
| mws1474 | 344.09 | [M+H]+ | 5,7-Dihydroxy-3′,4′,5′-trimethoxyflavone | Flavones | 5.22 | 0.93 / | 0.56 / | 5.11 | 0.98 / |
| pmp001076 | 372.12 | [M+H]+ | Isosinensetin * | Flavones | 0.88 / | 1.99 / | 0.22 | 0.62 / | 0.70 / |
| mws0043 | 402.13 | [M+H]+ | Nobiletin | Flavones | 0.83 / | 6.91 | 0.11 | 0.77 / | 0.93 / |
| pmn001668 | 416.15 | [M−H]− | Apigenin-3-O-rhamnoside | Flavones | 1.56 / | 2.28 | 1.74 / | 1.40 / | 0.90 / |
| Lmlp005572 | 432.11 | [M+H]+ | Galangin-7-O-glucoside * | Flavones | 2.72 | 1.64 / | 1.45 / | 0.61 / | 0.22 |
| HJN076 | 564.18 | [M−H]− | (2R)-Pinocembrin-7-O-neohesperidoside | Flavones | 5.08 | 2.97 | 1.76 / | 4.32 | 0.85 / |
| Lmnp002448 | 640.16 | [M+H]+ | 5,7,3′,4′-Tetrahydroxy-6-methoxyflavone-8-C-[glucosyl-(1-2)]-glucoside | Flavones | 1.05 / | 0.35 | 0.77 / | 0.42 | 0.40 |
| Lmgn002843 | 286.05 | [M−H]− | 2′-Hydroxyisoflavone | Isoflavones | 1.97 / | 0.73 / | 1.63 / | 0.80 / | 0.41 |
| mws1068 | 286.05 | [M−H]− | Kaempferol * | Flavonols | 2.03 | 0.43 | 1.55 / | 0.94 / | 0.46 |
| pme3514 | 302.04 | [M−H]− | Morin * | Flavonols | 3.40 | 0.50 / | 1.39 / | 1.45 / | 0.43 |
| pme2954 | 302.04 | [M+H]+ | Quercetin | Flavonols | 3.18 | 0.52 / | 1.20 / | 1.45 / | 0.46 |
| mws0038 | 314.08 | [M−H]− | Kumatakenin | Flavonols | 2.57 | 1.58 / | 0.42 / | 2.69 | 1.05 / |
| mws0917 | 330.07 | [M−H]− | 3,7-Di-O-methylquercetin | Flavonols | 5.31 | 0.46 | 2.02 | 1.40 / | 0.26 |
| pmp000365 | 370.11 | [M+H]+ | Uralenol | Flavonols | 0.48 | 0.91 / | 1.19 / | 0.63 / | 1.32 / |
| mws0055 | 372.12 | [M+H]+ | Tangeretin | Flavonols | 1.15 / | 8.18 | 0.07 | 0.83 / | 0.72 / |
| Lmmn003398 | 490.11 | [M−H]− | Kaempferol-3-O-(6″-acetyl)glucoside | Flavonols | 2.03 | 1.35 / | 1.21 / | 1.56 / | 0.77 / |
| Lmln001951 | 596.14 | [M−H]− | Quercetin-3-arabinosylglucoside * | Flavonols | 1.61 / | 0.84 / | 1.10 / | 0.70 / | 0.44 |
| pme1540 | 624.17 | [M+H]+ | Isorhamnetin-3-O-neohesperidoside | Flavonols | 1.08 / | 0.55 / | 0.43 | 0.43 | 0.40 |
| Hmcp001578 | 640.16 | [M+H]+ | Isorhamnetin-3,7-O-diglucoside * | Flavonols | 0.78 / | 0.99 / | 0.59 / | 0.50 | 0.64 / |
| Hmln001682 | 668.16 | [M−H]− | Quercetin-3-O-(2″-acetyl)-glucosylgalactoside | Flavonols | 18.83 | 0.73 / | 1.04 / | 6.54 | 0.35 |
| Hmcp001629 | 696.15 | [M+H]+ | Kaempferol-3-O-(6″-Malonylglucoside)-7-O-Glucoside | Flavonols | 7.97 | 2.67 | 1.12 / | 1.93 / | 0.24 |
| pmb0709 | 712.15 | [M+H]+ | Quercetin-7-O-malonylglucosyl-glucoside * | Flavonols | 4.98 | 1.39 / | 1.56 / | 2.92 | 0.59 / |
| pmb0706 | 712.15 | [M+H]+ | Quercetin-5-O-malonylglucosyl-glucoside | Flavonols | 9.37 | 1.05 / | 0.83 / | 3.50 | 0.37 |
| Hmcp001757 | 756.21 | [M+H]+ | Quercetin-O-rhamnoside-O-glucoside-O-rhamnoside | Flavonols | 3.26 | 0.89 / | 0.61 / | 1.19 / | 0.36 |
| mws1422 | 274.08 | [M+H]+ | Epiafzelechin * | Flavanols | 4.22 | 0.89 / | 0.74 / | 0.81 / | 0.19 |
| pmn001415 | 452.11 | [M−H]− | Catechin-(7,8-bc)-4β-(3,4-dihydroxyphenyl)-dihydro-2-(3H)-ne * | Flavanols | 0.94 / | 2.01 | 3.10 | 1.44 / | 1.53 / |
| pmn001416 | 452.11 | [M−H]− | Catechin-(7,8-bc)-4α-(3,4-dihydroxyphe-nyl)-dihydro-2-(3H)-ne | Flavanols | 1.09 / | 1.70 / | 3.02 | 1.47 / | 1.35 / |
| HJN041 | 452.13 | [M−H]− | Epicatechin glucoside | Flavanols | 1.12 / | 0.81 / | 0.94 / | 0.46 | 0.41 |
| Smlp002532 | 419.10 | [M]+ | Cyanidin-3-O-arabinoside | Anthocyanins | 4.44 | 1.33 / | 1.52 / | 0.97 / | 0.22 |
| pmb0550 | 449.11 | [M]+ | Cyanidin-3-O-glucoside (Kuromanin) | Anthocyanins | 17.48 | 2.07 | 1.39 / | 1.36 / | 0.08 |
| pmb0542 | 535.11 | [M]+ | Cyanidin-3-O-(6″-Malonylglucoside) | Anthocyanins | 16.81 | 6.33 | 1.78 / | 1.91 / | 0.11 |
| pme1793 | 595.17 | [M]+ | Pelargonidin-3,5-diglucoside | Anthocyanins | 1.76 / | 0.46 | 2.08 | 0.51 / | 0.29 |
| Lmpp003815 | 625.16 | [M]+ | Petunidin-3-(6″-p-Coumaroylglucoside) | Anthocyanins | 0.98 / | 0.58 / | 0.40 | 0.48 | 0.49 |
| Lmjp001323 | 743.20 | [M]+ | Cyanidin 3-O-sambubioside-5-O-glucoside | Anthocyanins | 1.19 / | 0.83 / | 1.19 / | 0.49 | 0.41 |
| mws0024 | 170.02 | [M−H]− | Gallic acid | Proanthocyanidins | 2.66 | 0.51 / | 1.17 / | 1.40 / | 0.53 / |
| pmn001519 | 336.05 | [M−H]− | Galloyl Methyl gallate | Proanthocyanidins | 0.78 / | 0.75 / | 1.01 / | 0.50 | 0.64 / |
| Cmsn000894 | 362.08 | [M−H]− | 7-O-Galloyl-D-sedoheptulose | Proanthocyanidins | 1.52 / | 1.38 / | 1.41 / | 2.01 | 1.32 / |
| Wmhn001495 | 484.08 | [M−H] − | 1,4-Di-O-galloyl-glcose | Proanthocyanidins | 1.34 / | 0.97 / | 0.82 / | 0.64 / | 0.47 |
| Lmfn001209 | 636.10 | [M−H]− | 1,3,6-Tri-O-galloyl-D-glucose * | Proanthocyanidins | 1.82 / | 1.30 / | 0.76 / | 0.47 | 0.26 |
| Cmhn000855 | 650.08 | [M−H]− | Valoneoyl-glucose | Proanthocyanidins | 1.39 / | 1.63 / | 1.39 / | 3.36 | 2.42 |
| pme0432 | 576.13 | [M−H]− | Procyanidin A2 * | Proanthocyanidins | 2.07 | 0.70 / | 1.02 / | 0.73 / | 0.35 |
| pme0430 | 576.13 | [M−H]− | Procyanidin A1 | Proanthocyanidins | 2.06 | 1.13 / | 1.56 / | 1.21 / | 0.59 / |
| pmn001667 | 578.14 | [M−H]− | Procyanidin B4 * | Proanthocyanidins | 1.03 / | 1.07 / | 2.11 | 0.60 / | 0.58 / |
| HJN074 | 592.16 | [M−H]− | Procyanidin A6 | Proanthocyanidins | 0.66 / | 1.50 / | 0.86 / | 1.37 | 2.08 |
* Indicate there are several different isomerides of the marked compounds. / means not changed.
Moreover, the total content of six types of flavonoids in different samples was analyzed based on the peak area of each compound. As shown in Figure 3C, flavonols were the most abundant, which also had the largest number; they slightly increased in S2 and S3 rose petals and continued to rise in S4 petals. There was no obvious difference between S2 and S2D, but compared to S1 petals, S2D petals had a little higher content of flavonols. Proanthocyanidins were the second abundant in content, which increased in S2 petals, then slightly descended in S3 flowers and remained unchanged in S4 but showed no change in S2D compared to S1. As the second-largest number of flavonoid metabolites was detected in rose petals, flavones content also saw a sustained increase when exposed to light; however, it declined under shading light in S2D. Furthermore, the content of anthocyanin ceased to increase under shading, but continued to rise with illumination. Flavaonols exhibited no significant changes during rose flower development under both treatments. Isoflavones were the lowest in quantity and content, and they showed a declining trend from S1 to S3, then a slight rise at S4; however, they were expressed higher in S2D than that in S2 petals, but lower than S1 petals (Figure 3C).
4. Discussion
The ‘Chen Xi’ rose, whose petal colors can be changed during the flowering process, has highly ornamental value. Previous studies reported that the yellow color of the rose is determined by carotenoids, while the red color is mainly caused by anthocyanins [3,10][3][10]. Furthermore, rose petals have been widely used as traditional medicinal therapy, food additives, as well as for cosmetics due to these abundant bioactive compounds [4,11,16][4][11][16]. Here, we detected the flavonoid metabolites during different developmental stages of this bicolored rose flower, with one stage (S2) subjected to shading treatment.4.1. Abundant Flavonoid Metabolites Accumulated in Rose Flower Petals
In total, 176 flavonoid metabolites were identified, containing 49 flavones, 59 flavonols, 12 flavanones, 3 isoflavones, 12 anthocyanins, and 41 proanthocyanidins in this bicolored rose flower (Table S1). Among them, many metabolites were found for the first time. Previous studies identified four anthocyanin compounds, including cyanidin 3-O-glucoside, pelargonidin 3-glucoside, cyanidin 3,5-di-O-glucoside and pelargonidin 3,5-di-O-glucoside [10,11[10][11][12],12], which belongs to single or di-glycoside derivatives of cyanidin and pelargonidin. However, in this study, only two anthocyanins were the same as before (cyanidin-3-O-glucoside and pelargonidin-3,5-diglucoside), and five other cyanidins and one petunidin were glycosylated by other types of glycosides, including arabinoside, malonylglucoside, sambubioside, and coumaroylglucoside with single or diosaccharide. Other kinds of anthocyanin compounds with different glucosides were detected, such as delphinidin-3-O-glucoside, malvidin-3-O-malonylglucoside and petunidin-3-(6″-p-Coumaroylglucoside) (Table S1). Only 20 flavones, including 16 kaempferol and derivatives and 3 quercetins and derivatives as well as one flavan-3-ol derivative, were identified before [10]; however, 59 flavonols and other flavonoid compounds were detected (Table S1) which border the flavonoid metabolites in rose flowers. These results showed that rose flowers are rich in flavonoid metabolites, with their content being high during the blooming stages. Flavonoids, especially the high content of flavonols in rose flowers, were reported to have several bioactivities, such as antioxidant, anti-inflammatory, and anticancer roles [17,18,19][17][18][19]. Moreover, yellow rose flowers contain abundant carotenoids [10], which play vital roles in human and animal nutrition and in reducing the risk of vitamin A deficiency, cancer, and cardiovascular diseases [20,21][20][21]. Therefore, ‘Chen Xi’ flowers may have a higher medicine value for being rich in both flavonoids and carotenoids.
4.2. Light Affects Certain Flavonoid, Especially Anthocyanin, Biosynthesis of Rose Flowers
Light is important for plant growth and photomorphogenesis, and it affects the accumulation of pigment in plant organs, including fruit skin, such as pear [13[13][22],22], grape [15], strawberry [23], and apple [14] and flower petals, such as Lily [24], Lisianthus [25], Gerbera [26]. These organs’ color is due to anthocyanin pigment whose biosynthesis is dependent on light induction.
In this study, the rose flower bud was yellow, but the part which was exposed to sunlight turned red, and the petals become redder along with the blooming stage after it totally opens, shadowing the yellow color. However, when the flower was grown under the shade, the flower remained yellow, which was consistent with anthocyanin accumulation. Therefore, it can be inferred that light promotes anthocyanin accumulation in rose petals, but cannot affect carotenoids biosynthesis. Furthermore, the light also affects other flavonoid compounds’ biosynthesis, since most of them increased with the rose flower development under sunlight. It is a fantastic phenomenon, and we will identify this hypothesis from gene expression level in the next step, and study how light induces anthocyanin biosynthesis. It will be an interesting subject worth exploring further.
References
- Guoliang, W. History of roses in cultivation|Ancient Chinese Roses. Encycl. Rose Sci. 2003, 387–395. Available online: https://coek.info/queue/pdf-history-of-roses-in-cultivation-ancient-chinese-roses-.html (accessed on 23 September 2021).
- Raymond, O.; Gouzy, J.; Just, J.; Badouin, H.; Verdenaud, M.; Lemainque, A.; Vergne, P.; Moja, S.; Choisne, N.; Pont, C.; et al. The Rosa genome provides new insights into the domestication of modern roses. Nat. Genet. 2018, 50, 772–777.
- Wan, H.H.; Yu, C.; Han, Y.; Guo, X.; Ahmad, S.; Tang, A.; Wang, J.; Cheng, T.; Pan, H.; Zhang, Q. Flavonols and carotenoids in yellow petals of rose cultivar (rosa ‘sun city’): A possible rich source of bioactive compounds. J. Agric. Food Chem. 2018, 66, 4171–4181.
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F. Studies on chemical constituents and bioactivity of Rosa micrantha: An alternative antioxidants source for food, pharmaceutical, or cosmetic applications. J. Agric. Food Chem. 2010, 58, 6277–6284.
- Héthelyi, B.; Szarka, S.; Lemberkovics, É.; Szke, É. SPME-GC/MS identification of aroma compounds in rose flowers. Acta Agron. Hung. 2010, 58, 283–287.
- Ibrahim, M.; Du, X.; Agarwal, M.; Hardy, G.; Abdulhussein, M.; Ren, Y. Influence of benzyladenine on metabolic changes in different rose tissues. Plants 2018, 7, 95.
- Prata, G.G.; Souza, K.O.D.; Lopes, M.M.D.; Aragão, F.A.S.D.; Oliveira, L.D.S.; Alves, R.E.; Silva, S. Nutritional characterization, bioactive compounds and antioxidant activity of brazilian roses (Rosa spp.). J. Agric. Sci. Technol. 2017, 19, 929–941.
- Nanjaraj, U.A.N.; Hu, Y.; Li, P.; Michael, Y.; Chen, Y.; Zhang, Y. Cloning and Expression of a Nonribosomal Peptide Synthetase to Generate Blue Rose. ACS Synth. Biol. 2019, 8, 1698–1704.
- Schmitzer, V.; Veberic, R.; Osterc, G.; Strampar, F. Color and phenolic content changes during flower development in groundcover rose. J. Am. Soc. Hortic. Sci. 2010, 135, 195–202.
- Wan, H.; Chao, Y.; Han, Y.; Guo, X.; Luo, L.; Pan, H.; Zheng, T.; Wang, J.; Cheng, T.; Zhang, Q. Determination of flavonoids and carotenoids and their contributions to various colors of rose cultivars (Rosa spp.). Front. Plant Sci. 2019, 10, 123.
- Lee, J.H.; Lee, H.; Choung, M. Anthocyanin compositions and biological activities from the red petals of Korean edible rose (Rosa hybrida cv. Noblered). Food Chem. 2011, 129, 272–278.
- Huang, P.; Lin, F.; Li, B.; Zheng, Y. Hybrid-Transcriptome sequencing and associated metabolite analysis reveal putative genes involved in flower color difference in rose mutants. Plants 2019, 8, 267.
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Yan, X.; Yang, Q.; Wu, Z.; Zeng, Y.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997.
- Fang, H.; Dong, Y.; Yue, X.; Hu, J.; Jiang, S.; Xu, H.; Wang, Y.; Su, M.; Zhang, J.; Zhang, Z.; et al. The B-box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant Cell Environ. 2019, 42, 2090–2104.
- Sun, L.; Li, S.; Tang, X.; Fang, X.; Zhang, Y.; Jiang, J.; Liu, J.; Liu, C. Transcriptome analysis reveal the putative genes involved in light-induced anthocyanin accumulation in grape ‘Red Globe’ (V. vinifera L.). Gene 2020, 728, 144284.
- Sarangowa, O.; Kanazawa, T.; Nishizawa, M.; Myoda, T.; Bai, C.; Yamagishi, T. Flavonol glycosides in the petal of Rosa species as chemotaxonomic markers. Phytochemistry 2014, 107, 10761–10768.
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245.
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534.
- Zhang, S.; Lu, B.; Han, X.; Xu, L.; Qi, Y.; Yin, L.; Xu, Y.; Zhao, Y.; Liu, K.; Peng, J. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2019, 132, 110695.
- Aydemir, G.; Kasiri, Y.; Bartók, E.M.; Birta, E.; Fröhlich, K.; Böhm, V.; Mihaly, J.; Rühl, R. Lycopene supplementation restores vitamin A deficiency in mice and possesses thereby partial pro-vitamin A activity transmitted via RAR signaling. Mol. Nutr. Food Res. 2016, 60, 2413–2420.
- Di Pietro, N.; Di Tomo, P.; Pandolfi, A. Carotenoids in cardiovascular disease prevention. JSM Atheroscler. 2016, 1, 1.
- Bai, S.; Tao, R.; Yin, L.; Ni, J.B.; Yang, Q.S.; Yan, X.H.; Yang, F.; Guo, X.P.; Li, H.X.; Teng, Y.W. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit. Plant J. 2019, 100, 1208–1223.
- Xu, P.; Christopher, Z.; Li, Y.; Wu, J.; Liu, L.; Liu, Z.; Cai, R.; Lian, H. Transcriptome sequencing reveals role of light in promoting anthocyanin accumulation of strawberry fruit. Plant Growth Regul. 2018, 86, 121–132.
- Yamagishi, M. A novel R2R3-MYB transcription factor regulates light-mediated floral and vegetative anthocyanin pigmentation patterns in Lilium regale. Mol. Breed. 2016, 36, 1.
- Meir, S.; Kochanek, B.; Glick, A. Reduced Petal Pigmentation in Lisianthus (Eustoma grandiflorum) Flowers under low light conditions is associated with decreased expression of anthocyanin biosynthesis genes. Acta Hortic. 2010, 877, 1735–1744.
- Meng, X.; Peng, J.; Wang, X. Anthocyanin Accumulation and CHS, DFR Gene expression regulated by light and sugar in Gerbera hybrida Ray Floret. Acta Hortic. Sin. 2007, 34, 227–230.
More
