Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Mi Nguyen-Tra Le.
Staphylococcus aureus is a bacterium that mainly colonizes the nasal cavity and skin. To colonize the host, it is necessary for S. aureus to resist many antibacterial factors derived from human and commensal bacteria. Among them are the bacteria-derived antimicrobial peptides (AMPs) called bacteriocins. It was reported that some two-component systems (TCSs), which are signal transduction systems specific to bacteria, are involved in the resistance to several bacteriocins in S. aureus. However, the TCS-mediated resistance is limited to relatively low concentrations of bacteriocins, while high concentrations of bacteriocins still exhibit antibacterial activity against S. aureus.
- antimicrobial peptides
- bacteriocin
- two-component system
1. Introduction
Commensal bacteria inhabit the parts of the human body that come in contact with the external environment (oral cavity, digestive organs, vagina, anus, skin, etc.). Commensal bacteria compete and cooperate with each other in the environment. In organs where microorganisms are originally resident, the number of bacteria is controlled by the immune system to prevent infectious diseases. However, in the compromised hosts such as elderly people and patients with systemic diseases, the immune activity in these individuals is considered to be weakened. Under such conditions, the proportion of each bacterial species in some sites of the human body is altered, causing dysbiosis; in some cases, infectious diseases occur. Antibiotics (currently called “antibacterial chemotherapeutic agents”) are used to treat bacterial infections. However, depending on the dose and frequency of antibiotic administration, drug-resistant bacteria sometimes emerge.
Staphylococcus aureus is known as a commensal bacterium in humans; it generally localizes in the nasal cavity, skin, and intestine. S. aureus is a highly adaptable bacterium causing opportunistic infections, such as suppurative diseases, pneumonia, and sepsis [1,2,3][1][2][3]. Additionally, S. aureus causes food poisoning because it produces several heat-stable enterotoxins [2]. S. aureus is a pathogenic bacterium with a wide variety of virulence factors, and antibiotic resistance is likely to occur with long-term exposure to antibacterial chemotherapeutic agents [4]. According to the 2013 Centers for Disease Control and Prevention (CDC) report, 80,000 people were affected by methicillin-resistant Staphylococcus aureus (MRSA) in the USA in that year. In addition, the O’Neill Report [5] estimated that the number of deaths from drug-resistant bacteria would exceed that from cancer in 2050. Among the infectious disease-causing microorganisms listed in this report are various drug-resistant bacteria, such as MRSA, penicillin-resistant Streptococcus pneumoniae (PRSP), and carbapenem-resistant enterobacteriaceae (CRE). In response to these reports, counterplans against drug-resistant bacteria, known as antimicrobial resistance (AMR) action plans, are advocated worldwide.
Antibacterial chemotherapeutic agents are generally administered to cure S. aureus infections. However, the emergence of MRSA has become a growing challenge of this treatment approach. Additionally, disinfectants are also widely used for the prevention of nosocomial infection. It is reported that the qac genes, which encodes a multidrug efflux pump that expels toxic molecules, contributes to the development of resistance to quaternary ammonium compounds such as benzalkonium chloride [6,7][6][7]. In the genus Staphylococcus, the qac genes are encoded in a plasmid, and six types of Qac efflux pumps are reported. Among the Qac proteins, QacA and QacB are highly conserved among Staphylococcus species, while QacC, QacG, QacH, and QacJ, which belong to the small multidrug resistance (SMR) family, are known to have amino acid sequence diversity among the Staphylococcus species. Therefore, S. aureus shows resistance not only to several antibacterial chemotherapeutic agents but also to the other antibacterial agents such as disinfectants. In recent years, antimicrobial peptides (AMPs) have attracted attention as antibacterial chemotherapeutic agents. These AMPs are derived from various living organisms, such as humans, plants, and bacteria [8,9,10,11][8][9][10][11]. Bacterial AMPs are also called bacteriocins. Some of these antibacterial peptides and bacteriocins were also shown to be effective against MRSA [12,13,14][12][13][14] and have potential applications in the clinic [15,16][15][16]. Therefore, these peptides are attracting attention as candidates for next-generation antibacterial chemotherapeutic agents because of their high stability and the establishment of purification methods in recent years.
2. Bacteriocins
Bacteriocins are ribosomally synthesized peptides or proteins that exhibit antibacterial activity against bacterial species that are closely related to bacteriocin producers [17,18][17][18]. Bacteriocins are mainly classified into classes I and II [19]. Class I bacteriocins (peptides <5 kDa) are called “lantibiotics” and contain a ring bridged by lanthionine and 3-methyllanthionine residues [20], whereas class II bacteriocins comprise unmodified amino acids [20]. Lantibiotics are subdivided into types A and B [21]. Type A lantibiotics bind to lipid II, which is involved in peptidoglycan synthesis, and then inhibit cell wall biosynthesis and disturb the bacterial membrane [17[17][20],20], while type B lantibiotics are globular peptides that inhibit cell wall biosynthetic steps such as transglycosylation [22]. Type A lantibiotics are further classified into two subtypes: type A(I), while lactin 481 and nukacin ISK-1 are classified as subtypes of type A(II) [19]. Class II bacteriocins are classified into the following three subclasses: IIa, IIb, and IIc [23].
Nisin A is a bacteriocin produced by L. lactis [24]. Nisin A is a lantibiotic that contains unusual amino acids such as lanthionine, β-methyllanthionine, and dehydrated amino acids [20]. Nisin A binds to lipid II, resulting in membrane disturbance. Recently, it was reported that nisin A is associated with DNA condensation by interfering with chromosome replication or segregation in S. aureus [25]. Nisin A has broad-spectrum antimicrobial activity, mainly against gram-positive bacteria [26,27,28,29,30,31][26][27][28][29][30][31]. Due to its broad-spectrum activity, nisin A is widely used as a food additive worldwide for the prevention of food poisoning [26,32,33][26][32][33]. In addition, Alves DCB et al. reported the potential use of nisin combined with oxacillin for methicillin-resistant S. aureus [34]. Bacteriocins, including nisin A, were also investigated as potential antibacterial chemotherapeutic agents for clinical application [26,29,35][26][29][35].
3. Two-Component Systems and Their Association with AMP Resistance
Recently, two-component systems (TCSs) were reported to be associated with the resistance to several types of antibacterial agents, such as bacitracin, vancomycin, human β defensins (hBDs), LL37, and bacteriocins [36,37,38,39,40,41][36][37][38][39][40][41]. TCSs are predominantly found in prokaryotes. TCSs comprise a sensory histidine kinase (HK) and a cognate response regulator (RR) [42,43][42][43]. The sensor is a transmembrane protein that senses changes in the external environment, resulting in autophosphorylation of histidine residues (HKs) in the sensor and transfer the phosphate to aspartate residues of the cognate response regulator (RR) [43,44][43][44]. The phosphorylated RR then binds to target DNA elements with strong affinity, activating or repressing the transcription of target genes. Thus, bacteria are able to quickly adapt to the external environment by regulating the expression of the respective genes.
It was revealed that S. aureus has 16 sets of TCSs. The function of each TCS is shown in Table 1. A well-studied TCS is the Agr system, which is known to be widely involved in the regulation of virulence factor expression. Agr has a central role in the quorum-sensing system, which senses cell density via autoinducer peptides (AIPs) [45]. Agr is involved in the expression of many factors, including virulence factors mediated by RNAIII, a gene product of hld (delta-hemolysin). RNAIII was demonstrated to directly upregulate hla (α-haemolysin) expression [45] and downregulate the expression of spa (protein A) [46] and the transcription factor rot gene, which is responsible for the repression of toxins [47]. RNAIII binds to the target mRNA directly, resulting in the up- or down-regulation of gene expression. Phenol soluble modulins (PSMs) were demonstrated to be regulated by AgrA directly and have versatile virulence activities such as epithelial colonization, cytotoxic activity, biofilm formation, and antimicrobial activity [48,49,50][48][49][50]. However, the precise mechanism of the expression of other virulence factors mediated by the Agr system is still unknown. SaeRS, one of the TCSs in S. aureus, is also known as a global regulator of virulence factors and is important for the regulation of coagulase, α-toxin, β-haemolysin, γ-haemolysin, staphylococcal immunoglobulin-binding protein, nuclease, leucocidin, toxic shock syndrome toxin-1 (TSST-1), epidermis deprivation toxin, etc. SaeRS promotes the expression of coagulase [51,52,53][51][52][53].
Table 1. Function of two-component system in Staphylococcus aureus MW2 strain.
| TCS no. | Gene Name | Gene ID | Function |
|---|---|---|---|
| TCS1 | vicRS | MW0018-19 | Cell division/separation, lethal |
| TCS2 | htpRS | MW0198-99 | response to extracellular phosphates and survival/multiplication within host cells |
| TCS3 | lytSR | MW0236-37 | Lytic enzymes |
| TCS4 | apsRS, graRS | MW0621-22 | Bacterial surface charge, dltABCD, mprF |
| TCS5 | saeRS | MW0667-68 | Virulence factor expression |
| TCS6 | - | MW1208-09 | No report |
| TCS7 | arlRS | MW1304-05 | Virulence factor expression |
| TCS8 | srrAB | MW1445-46 | Oxidative stress |
| TCS9 | phoPR | MW1636-37 | inorganic phosphate uptake |
| TCS10 | - | MW1789-90 | No report |
| TCS11 | vraSR | MW1824-25 | Resistant against cell wall synthesis inhibitor |
| TCS12 | agrCA | MW1962-63 | quorum sensing, virulence factor expression |
| TCS13 | kdpDE | MW2002-03 | Neutrophil sensitivity |
| TCS14 | hssRS | MW2282-83 | Iron efflux |
| TCS15 | nreCB | MW2313-14 | nitrate respiration |
| TCS16 | bceRS, braRS | MW2544-45 | Bacteriocin resistance |
TCSs were reported to be involved in controlling the susceptibility to human-derived AMPs. AMPs are innate immune factors and are produced in various tissues and organs, such as the skin, lungs, and intestines [8,9,10,11,54][8][9][10][11][54]. The most well-known AMPs are defensins. Defensins are classified into two types: α-defensins from neutrophils and Paneth cells and β-defensins (hBDs) from mainly epithelial cells [8,10,55][8][10][55]. Another major peptide is human cathelicidin (LL37), which is found in various cells, including neutrophils and epithelial cells [10]. The Aps system is related to resistance against human β-defensin-3 (hBD3), LL37, and bacteriocins (nisin A, nukacin ISK-1), which possess a strong positive charge. ApsR regulates dlt and mprF (fmtC) expression, causing an increase in cell surface charge [36,41,56][36][41][56]. Dlt is involved in alanine addition to teichoic acids in cell walls, while MprF is involved in lysine addition to phosphatidylglycerol in cell membranes [57,58][57][58]. Alanylation of teichoic acids and lysyl-phosphatidylglycerol contribute a shift to a weak negative charge on the cell surfaces (Figure 1). Since apsRS expression is negatively controlled by Agr, this resistance system is mainly observed in the early stage of bacterial growth with low expression of Agr, while in the stationary phase, Agr expression is increased, leading to suppression of the expression of ApsRS. Therefore, the charge on the surface of the bacterial cells is altered during growth. As a result, the susceptibility to antibacterial peptides changes during growth, with low susceptibility observed in the exponential phase and high susceptibility in the stationary phase [37].
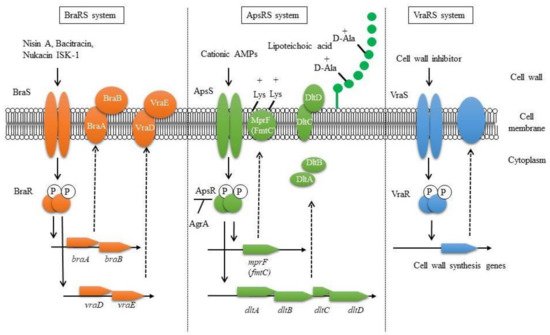

Figure 1. Proposed bacteriocin resistance mechanism mediated by TCSs in S. aureus.
VraSR regulates many factors involved in cell wall biosynthesis and is associated with the susceptibility to cell wall synthesis inhibitors such as β-lactams, vancomycin, cycloserine, teicoplanin, and bacitracin [38,39,59][38][39][59]. Upon the addition of cell wall synthesis inhibitors, VraSR is activated, resulting in the upregulation of several cell wall synthesis genes, including the transpeptidase pbp2 and the transglycosylase sgtB [38].
BraRS, which is involved in the acquisition of bacteriocin resistance, was first discovered to be involved in the resistance to bacitracin, one of the bacteriocins produced by Bacillus subtilis [60]. The resistance mechanism involves the sensing of low concentrations of bacitracin by the complex of the BraRS TCS and the upstream ABC transporter BraDE. As a result, the regulator BraR promotes the expression of the ABC transporter VraDE, an intrinsic resistance factor for bacitracin [61]. The BraRS-VraDE system is considered to be a TCS system that widely supports the sensing of several bacteriocins because it is also involved in the resistance to nukacin ISK-1 produced by Staphylococcus warneri and nisin A produced by L. lactis [40,41][40][41]. In addition, it was reported that the ABC transporter BraDE, encoded with BraRS regulon, was also associated with nisin resistance by directly interacting with BraS [62].
In conclusion, regarding bacteriocin resistance, it was clarified that BraRS regulates the expression of ABC transporters to promote resistance against several bacteriocins. ApsRS and VraRS also participate in bacteriocin resistance by changing the charge and increasing the expression of cell wall synthesis genes, respectively (Figure 1) [41]. In this way, it is expected that S. aureus performs precise TCS-mediated control to survive even in the presence of many bacteriocins produced by some other bacteria colocalized in the bacterial flora.
Three TCSs are known to be involved in bacteriocin resistance: BraRS, ApsRS, and VraRS. BraRS is a TCS that senses various bacteriocins and induces the expression of the ABC transporter vraDE. BraAB is required for BraS to sense bacteriocins, and braAB expression is also induced via BraRS (left). ApsRS is involved in resistance to positively charged antimicrobial peptides (AMPs). Aps controls the cell surface charge by regulating the expression of dlt and mprF. Aps is negatively controlled by Agr, the quorum sensing system (middle). VraRS is involved in resistance to cell wall synthesis inhibitors and regulates several genes in the cell wall synthesis system such as pbp2, sgtB, and murZ (right).
Amino acid sequences of ApsRS and BraRS from S. aureus are compared with those from the other staphylococci (Figure 2 and Table 2). Although ApsS in S. aureus does not have a high similarity with that of the other staphylococci, the response regulator ApsR in S. aureus shows relatively high similarity (above 79% identity) with that of other staphylococcal species except for S. pseudintermedius. It is speculated that this system, which changes the surface charge, is widely conserved among staphylococci. On the contrary, BraRS in S. aureus shows low similarity with that of the other staphylococci. Therefore, BraRS, which senses nisin A, bacitracin, and Nukacin ISK-1, may be specific to S. aureus.
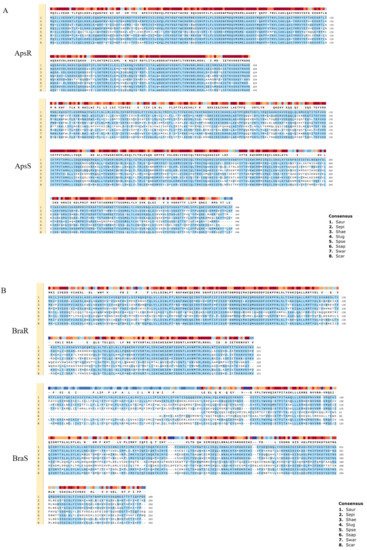

Figure 2. Comparison of two-component systems showing homology with ApsRS and BraRS of S. aureus among staphylococci. Alignment of ApsRS (A) of BraRS (B) among 8 staphylococcal species. 1. Staphylococcus aureus (Saur), 2. Staphylococcus epidermidis (Sepi), 3. Staphylococcus haemolyticus (Shae), 4. Staphylococcus lugdunensis (Slug), 5. Staphylococcus pseudintermedius (Spse), 6. Staphylococcus saprophyticus (Ssap), 7. Staphylococcus warneri (Swar), 8. Staphylococcus carnosus (Scar).
Table 2. % amino acid sequence identity of ApsRS and BraRS in S. aureus compared to eight Staphylococcal species.
| S. aureus MW2 | % Sequence Identity in: | ||||||
|---|---|---|---|---|---|---|---|
| S. epidermidis | S. haemolyticus | S. lugdunensis | S. pseudintermedius | S. saprophyticus | S. warneri | S. carnosus | |
| ApsR | 91.52 | 85.71 | 85.71 | 62.50 | 79.37 | 91.52 | 84.82 |
| ApsS | 69.67 | 67.73 | 67.05 | 46.47 | 60.00 | 73.70 | 69.08 |
| BraR | 79.64 | 78.28 | 76.47 | 42.53 | 41.82 | 81.45 | 78.28 |
| BraS | 60.34 | 63.01 | 58.56 | 30.17 | 28.68 | 62.80 | 61.64 |
References
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532.
- Manders, S.M. Toxin-mediated streptococcal and staphylococcal disease. J. Am. Acad. Dermatol. 1998, 39, 383–400.
- Foster, T.J. The Staphylococcus aureus “superbug”. J. Clin. Investig. 2004, 114, 1693–1696.
- Diep, B.A.; Otto, M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008, 16, 361–369.
- O’Neill, J. Review on Antimicrobial Resistance Antimicrobial Resistance: Tackling a Crisis For The Health and Wealth of Nations. London: Review on Antimicrobial Resistance. 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 21 September 2021).
- McDanel, J.S.; Murphy, C.R.; Diekema, D.J.; Quan, V.; Kim, D.S.; Peterson, E.M.; Evans, K.D.; Tan, G.L.; Hayden, M.K.; Huang, S.S. Chlorhexidine and mupirocin susceptibilities of methicillin-resistant Staphylococcus aureus from colonized nursing home residents. Antimicrob. Agents Chemother. 2013, 57, 552–558.
- Wassenaar, T.M.; Ussery, D.; Nielsen, L.N.; Ingmer, H. Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species. Eur. J. Microbiol. Immunol. 2015, 5, 44–61.
- Ganz, T.; Selsted, M.E.; Szklarek, D.; Harwig, S.S.; Daher, K.; Bainton, D.F.; Lehrer, R.I. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 1985, 76, 1427–1435.
- Ganz, T.; Lehrer, R.I. Defensins. Pharmacol. Ther. 1995, 66, 191–205.
- Lehrer, R.I.; Ganz, T. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 1999, 11, 23–27.
- Zaiou, M.; Gallo, R.L. Cathelicidins, essential gene-encoded mammalian antibiotics. J. Mol. Med. 2002, 80, 549–561.
- Karczewski, J.; Brown, C.M.; Maezato, Y.; Krasucki, S.P.; Streatfield, S.J. Efficacy of a novel lantibiotic, CMB001, against MRSA. J. Antimicrob. Chemother. 2021, 76, 1532–1538.
- Walsh, L.; Johnson, C.N.; Hill, C.; Ross, R.P. Efficacy of Phage- and Bacteriocin-Based Therapies in Combatting Nosocomial MRSA Infections. Front. Mol. Biosci. 2021, 8, 654038.
- Ouhara, K.; Komatsuzawa, H.; Kawai, T.; Nishi, H.; Fujiwara, T.; Fujiue, Y.; Kuwabara, M.; Sayama, K.; Hashimoto, K.; Sugai, M. Increased resistance to cationic antimicrobial peptide LL-37 in methicillin-resistant strains of Staphylococcus aureus. J. Antimicrob. Chemother. 2008, 61, 1266–1269.
- Bendjeddou, K.; Hamma-Faradji, S.; Meddour, A.A.; Belguesmia, Y.; Cudennec, B.; Bendali, F.; Daube, G.; Taminiau, B.; Drider, D. Gut microbiota, body weight and histopathological examinations in experimental infection by methicillin-resistant Staphylococcus aureus: Antibiotic versus bacteriocin. Benef. Microbes 2021, 12, 295–305.
- Mao, Y.; Hoffman, T.; Singh-Varma, A.; Duan-Arnold, Y.; Moorman, M.; Danilkovitch, A.; Kohn, J. Antimicrobial Peptides Secreted From Human Cryopreserved Viable Amniotic Membrane Contribute to its Antibacterial Activity. Sci. Rep. 2017, 7, 13722.
- Nissen-Meyer, J.; Nes, I.F. Ribosomally synthesized antimicrobial peptides: Their function, structure, biogenesis, and mechanism of action. Arch. Microbiol. 1997, 167, 67–77.
- Jack, R.W.; Tagg, J.R.; Ray, B. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 1995, 59, 171–200.
- Bierbaum, G.; Sahl, H.-G. Lantibiotics: Mode of action, biosynthesis and bioengineering. Curr. Pharm. Biotechnol. 2009, 10, 2–18.
- Nagao, J.; Asaduzzaman, S.M.; Aso, Y.; Okuda, K.-I.; Nakayama, J.; Sonomoto, K. Lantibiotics: Insight and foresight for new paradigm. J. Biosci. Bioeng. 2006, 102, 139–149.
- Nes, I.F.; Holo, H. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers 2000, 55, 50–61.
- Brötz, H.; Bierbaum, G.; Leopold, K.; Reynolds, P.E.; Sahl, H.G. The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob. Agents Chemother. 1998, 42, 154–160.
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788.
- Delves-Broughton, J.; Blackburn, P.; Evans, R.J.; Hugenholtz, J. Applications of the bacteriocin, nisin. Antonie Van Leeuwenhoek 1996, 69, 193–202.
- Jensen, C.; Li, H.; Vestergaard, M.; Dalsgaard, A.; Frees, D.; Leisner, J.J. Nisin Damages the Septal Membrane and Triggers DNA Condensation in Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 1007.
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465.
- Le Lay, C.; Dridi, L.; Bergeron, M.G.; Ouellette, M.; Fliss, I.L. Nisin is an effective inhibitor of Clostridium difficile vegetative cells and spore germination. J. Med. Microbiol. 2016, 65, 169–175.
- Udompijitkul, P.; Paredes-Sabja, D.; Sarker, M.R. Inhibitory effects of nisin against Clostridium perfringens food poisoning and nonfood-borne isolates. J. Food Sci. 2012, 77, M51–M56.
- Tong, Z.; Ni, L.; Ling, J. Antibacterial peptide nisin: A potential role in the inhibition of oral pathogenic bacteria. Peptides 2014, 60, 32–40.
- Okuda, K.; Zendo, T.; Sugimoto, S.; Iwase, T.; Tajima, A.; Yamada, S.; Sonomoto, K.; Mizunoe, Y. Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 2013, 57, 5572–5579.
- Field, D.; O’ Connor, R.; Cotter, P.D.; Ross, R.P.; Hill, C. In Vitro Activities of Nisin and Nisin Derivatives Alone and In Combination with Antibiotics against Staphylococcus Biofilms. Front. Microbiol. 2016, 7, 508.
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274.
- Juturu, V.; Wu, J.C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 2018, 36, 2187–2200.
- Alves, F.C.B.; Albano, M.; Andrade, B.F.M.T.; Chechi, J.L.; Pereira, A.F.M.; Furlanetto, A.; Rall, V.L.M.; Fernandes, A.A.H.; Dos Santos, L.D.; Barbosa, L.N.; et al. Comparative Proteomics of Methicillin-Resistant Staphylococcus aureus Subjected to Synergistic Effects of the Lantibiotic Nisin and Oxacillin. Microb. Drug Resist. 2020, 26, 179–189.
- Van Harten, R.M.; Willems, R.J.L.; Martin, N.I.; Hendrickx, A.P.A. Multidrug-Resistant Enterococcal Infections: New Compounds, Novel Antimicrobial Therapies? Trends Microbiol. 2017, 25, 467–479.
- Li, M.; Lai, Y.; Villaruz, A.E.; Cha, D.J.; Sturdevant, D.E.; Otto, M. Gram-positive three-component antimicrobial peptide-sensing system. Proc. Natl. Acad. Sci. USA 2007, 104, 9469–9474.
- Matsuo, M.; Oogai, Y.; Kato, F.; Sugai, M.; Komatsuzawa, H. Growth-phase dependence of susceptibility to antimicrobial peptides in Staphylococcus aureus. Microbiology 2011, 157, 1786–1797.
- Kuroda, M.; Kuroda, H.; Oshima, T.; Takeuchi, F.; Mori, H.; Hiramatsu, K. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 2003, 49, 807–821.
- Belcheva, A.; Golemi-Kotra, D. A close-up view of the VraSR two-component system. A mediator of Staphylococcus aureus response to cell wall damage. J. Biol. Chem. 2008, 283, 12354–12364.
- Kawada-Matsuo, M.; Yoshida, Y.; Nakamura, N.; Komatsuzawa, H. Role of two-component systems in the resistance of Staphylococcus aureus to antibacterial agents. Virulence 2011, 2, 427–430.
- Kawada-Matsuo, M.; Yoshida, Y.; Zendo, T.; Nagao, J.; Oogai, Y.; Nakamura, Y.; Sonomoto, K.; Nakamura, N.; Komatsuzawa, H. Three Distinct Two-Component Systems Are Involved in Resistance to the Class I Bacteriocins, Nukacin ISK-1 and Nisin A, in Staphylococcus aureus. PLoS ONE 2013, 8, e69455.
- Mitrophanov, A.Y.; Groisman, E.A. Signal integration in bacterial two-component regulatory systems. Genes Dev. 2008, 22, 2601–2611.
- Hoch, J.A. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 2000, 3, 165–170.
- Rampersaud, A.; Harlocker, S.L.; Inouye, M. The OmpR protein of Escherichia coli binds to sites in the ompF promoter region in a hierarchical manner determined by its degree of phosphorylation. J. Biol. Chem. 1994, 269, 12559–12566.
- Novick, R.P. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 2003, 48, 1429–1449.
- Huntzinger, E.; Boisset, S.; Saveanu, C.; Benito, Y.; Geissmann, T.; Namane, A.; Lina, G.; Etienne, J.; Ehresmann, B.; Ehresmann, C.; et al. Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J. 2005, 24, 824–835.
- Geisinger, E.; Adhikari, R.P.; Jin, R.; Ross, H.F.; Novick, R.P. Inhibition of rot translation by RNAIII, a key feature of agr function. Mol. Microbiol. 2006, 61, 1038–1048.
- Cheung, G.Y.C.; Joo, H.-S.; Chatterjee, S.S.; Otto, M. Phenol-soluble modulins--critical determinants of staphylococcal virulence. FEMS Microbiol. Rev. 2014, 38, 698–719.
- Peschel, A.; Otto, M. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 2013, 11, 667–673.
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.-H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-independent target gene control by the agr quorum-sensing system: Insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158.
- Giraudo, A.T.; Raspanti, C.G.; Calzolari, A.; Nagel, R. Characterization of a Tn551-mutant of Staphylococcus aureus defective in the production of several exoproteins. Can. J. Microbiol. 1994, 40, 677–681.
- Voyich, J.M.; Vuong, C.; DeWald, M.; Nygaard, T.K.; Kocianova, S.; Griffith, S.; Jones, J.; Iverson, C.; Sturdevant, D.E.; Braughton, K.R.; et al. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J. Infect. Dis. 2009, 199, 1698–1706.
- Baroja, M.L.; Herfst, C.A.; Kasper, K.J.; Xu, S.X.; Gillett, D.A.; Li, J.; Reid, G.; McCormick, J.K. The SaeRS Two-Component System Is a Direct and Dominant Transcriptional Activator of Toxic Shock Syndrome Toxin 1 in Staphylococcus aureus. J. Bacteriol. 2016, 198, 2732–2742.
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557.
- Cunliffe, R.N. Alpha-defensins in the gastrointestinal tract. Mol. Immunol. 2003, 40, 463–467.
- Li, M.; Cha, D.J.; Lai, Y.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 2007, 66, 1136–1147.
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Götz, F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 1999, 274, 8405–8410.
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A.; et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 2001, 193, 1067–1076.
- McCallum, N.; Meier, P.S.; Heusser, R.; Berger-Bächi, B. Mutational analyses of open reading frames within the vraSR operon and their roles in the cell wall stress response of Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 1391–1402.
- Yoshida, Y.; Matsuo, M.; Oogai, Y.; Kato, F.; Nakamura, N.; Sugai, M.; Komatsuzawa, H. Bacitracin sensing and resistance in Staphylococcus aureus. FEMS Microbiol. Lett. 2011, 320, 33–39.
- Hiron, A.; Falord, M.; Valle, J.; Débarbouillé, M.; Msadek, T. Bacitracin and nisin resistance in Staphylococcus aureus: A novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol. Microbiol. 2011, 81, 602–622.
- Randall, C.P.; Gupta, A.; Utley-Drew, B.; Lee, S.Y.; Morrison-Williams, G.; O’Neill, A.J. Acquired Nisin Resistance in Staphylococcus aureus Involves Constitutive Activation of an Intrinsic Peptide Antibiotic Detoxification Module. mSphere 2018, 3.
More
