Edible sprouts with germinating seeds of a few days of age are naturally rich in nutrients and other bioactive compounds. Among them, the cruciferous (Brassicaceae) sprouts stand out due to their high contents of glucosinolates (GLSs) and phenolic compounds.
- Brassicaceae
- elicitation
- growing conditions
- broccoli
- radish
- kale pak choi
- isothiocyanates
1. Introduction
2. Bioactive Secondary Metabolites in Edible Cruciferous Sprouts
As mentioned above, cruciferous sprouts contain non-nutrient/health-promoting compounds, such as diverse types of glucosinolates and phenolic compounds [5]. The biological activity developed by these compounds is mainly due to their antioxidant capacity, which could lower the deleterious consequences of excessively high levels of reactive oxygen species (ROS) in cells and, thus, decrease oxidative stress (OS) by providing cells with molecular tools to combat the imbalance between the production of ROS and the capacity to modulate the redox balance. These properties have direct effects on a number of cellular processes triggered by ROS, which are related to inflammation and oxidative reactions on DNA, proteins, and cell lipids [7]. In addition, to provide further molecular tools to cells to lower OS, many bioactive phytochemicals present in edible sprouts display biological functions that are crucial for the prevention of carcinogenesis processes and other chronic diseases [1] (Table 1).|
Edible Sprout |
Main Bioactive Compounds |
Elicitor Treatment Main Bioactivities Associated with Sprout Consumption |
Elicitor Classification References |
|||
|---|---|---|---|---|---|---|
Application |
Target Compound and Increase |
Reference |
||||
|
Broccoli (Brassica oleracea var. Italica) |
Flavonoids Quercetin, kaempferol, and flavonol glycosides |
|||||
|
Broccoli sprouts (Brassica oleracea) (7 days of growth) |
Sucrose, fructose, and glucose (146 mM) | Cancer risk (↓) Degenerative diseases (↓) Obesity-related metabolic disorders (↓) Allergic nasal symptoms (↓) Inflammation (↓) Pain (↓) Antioxidant capacity (↑) |
||||
Biotic elicitor |
In 0.5% agar media for 5 days after sowing seeds |
Total anthocyanins (10.0%) |
[28] |
Phenolic acids Chlorogenic, sinapic, and ferulic acid derivatives |
||
|
Broccoli sprouts (Brassica oleracea) (7 days of growth) |
Sucrose and mannitol (176 mM) |
Biotic elicitor |
Hydroponic system for 5 days after sowing seeds |
4. The Challenges of Including Cruciferous Sprouts in Balanced Diets and Personalized Nutrition
|
Matrix |
Pathophysiological Condition |
Effect |
Model |
Action Mechanism Z |
Ref. |
||||
|---|---|---|---|---|---|---|---|---|---|
|
Broccoli sprouts |
Metabolic profile |
No specific effect monitored |
Humans |
FA 14:1, FA 16:1, FA 18:1, FA 14:0, FA 16:0, FA 18:0, dehydroepiandrosterone, glutathione, cysteine, and glutamine (↑) Deoxy-uridin monophosohate (↓) |
[42] |
||||
|
Radish sprouts |
Energy metabolism |
Decrease glucose level | Total anthocyanins (40.0%) and phenolics (60.0%) Total glucosinolates (50.0%) |
Drosophila melanogaster |
Expression of spargel (↑) | [28] |
[43] | ||
|
Glucosinolates Glucoraphanin, glucoiberin, glucoraphenin, glucobrassicin, 4-hydroxyglucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin |
|||||||||
|
Broccoli (Brassica oleracea) (7 days of growth) |
Met (5 mM) Trp (10 mM) SA (100 μM) MeJA (25 μM) |
Biotic elicitors (Met, Trp, and plant hormones—SA and MeJA) |
Daily exogenous spraying during 3, 5, and 7 days |
Met: glucoiberin, glucoraphanin, and glucoerucin (30.0%) Trp: 4-hydroxyglucobrassicin, glucobrassicin, 4-Methoxyglucobrassicin, and neoglucobrassicin (80.0%) SA: 4-hydroxyglucobrassicin, glucobrassicin, 4-Methoxyglucobrassicin, and neoglucobrassicin (30.0%) MeJA: 4-hydroxyglucobrassicin, glucobrassicin, 4-Methoxyglucobrassicin, neoglucobrassicin (50.0%) |
[29] |
Isothiocyanates Sulphoraphane, iberin, and indole-3-carbinol |
|||
|
Broccoli sprouts (Brassica oleracea) |
Sucrose (146 mM) |
Biotic elicitor |
In 0.5% agar media for 5 days after sowing |
Total GLS (2.0-fold) |
[28] |
Radish (Raphanus sativus L.) |
|||
|
Broccoli sprouts (Brassica oleracea) (7 days of growth) |
Flavonoids Quercetin |
Mg (300 mg L−1) |
Risk of cancer (↓) Heart disease (↓) Diabetes (↓) Antioxidant capacity (↑) |
Abiotic elicitor | [9] |
||||
Suplementation with MgSO | 4 |
Increase of total ascorbic acid contain (29.1–44.5%) |
[27] |
Phenolic acids Ferulic, caffeic and p-coumaric acids, and derivatives |
|||||
|
Radish sprouts (raphanistrum subsp. sativus) (12 days of growth) |
MeJA (100 μM) |
Biotic elicitor (plant hormones—MeJA) |
Treatment with MeJA in growth chamber under dark conditions |
Glucoalyssin (1.4-fold) Glucoerucin (2.0-fold) Glucotropaeolin (1.8-fold) Glucoraphasatin (1.4-fold) |
[30] |
Glucosinolates Glucoraphenin, dehydroerucin, glucobrassicin, and 4-methoxyglucobrassicin |
|||
|
Radish sprouts (raphanistrum subsp. sativus) (12 days of growth) |
MeJA (100 μM) Light |
Biotic elicitor (plant hormones—MeJA-) Abiotic elicitor |
Treatment with MeJA in growth chamber under light |
Glucoraphanin (1.5-fold) Glucoerucin (1.6-fold) Glucotropaeolin (1.3-fold) 4-hydroxyglucobrassicin (4.4-fold) Pergonidin (1.7-fold) Cyanidin (2.0-fold) |
[30] |
Isothiocyanates Sulforaphene, sulforaphane, and indole-3-carbinol |
|||
|
Radish sprouts |
Mg (300 mg L−1) |
Abiotic elicitor |
Supplementation with MgSO4 |
Phenolic compounds (13.9–21.7%) |
[27] |
||||
Not determined | [ | ] |
Radish sprouts (raphanistrum subsp. sativus) |
NaCl (100 mM) |
Abiotic elicitor |
In 0.5% agar media for 3.5 and 7.0 days after sowing | |||
|
Rutabaga sprouts |
Thyroid function and iodine deficiency. Role as goitrogenic foods |
Protective effect against thyroid damage |
Total phenolics (30 and 50% in 5 and 7 day-old sprouts, respectively) Total GLS (50% and 120% in 5 and 7 day-old sprouts, respectively) |
Goitrogenic activity not discarded [31] |
|||||
Male rats |
Pak Choi sprouts (rapa subsp. chinensis) |
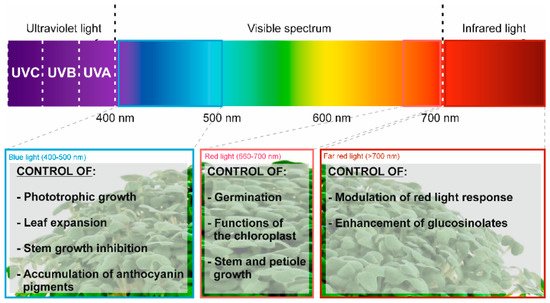
Application of different wavelengths of LED light (white, blue, and red) |
Abiotic elicitor |
Medium of perlite for 5 days in darkness and 18 h at the different wavelengths |
Total carotenoid content (12.1% and 9.2% with white light (respect to blue and red light, respectively) |
[25] |
|||
|
Pak Choi sprouts (rapa subsp. chinensis) |
Application of different wavelengths of LED light (white, blue, and red) |
Abiotic elicitor |
Medium of perlite for 5 days in darkness and 18 h at the different wavelengths |
Enhanced transcription of genes involved in carotenoid biosynthesis (CYP97A3, CYP97C1, βLCY, εLCY, β-OHASE1, PDS, PSY, VDE, ZEP) |
[25] |
||||
|
Kale Sprouts (oleracea var. sabellica) |
Application of different light wavelengths (470, 660, and 730 nm) |
Abiotic elicitor |
Seeds stratified for 2 days, exposed to light for 1 h, exposed to darkness for between 1 and 3 days and later, the specific light treatment |
Total GLS content (31.7%) |
[32 | ||||
|
Broccoli sprouts |
Pregnancy |
Prevention of brain injury in newborns |
Rats |
Not determined |
[44] |
||||
|
Broccoli sprouts |
Inflammation and oxidative stress |
Modulation of inflammation and vascular events |
Humans |
Not determined |
[45] |
||||
|
Broccoli sprouts |
Inflammation in overweight population |
Anti-inflammatory activity |
Humans |
IL-6 and C-reactive protein (↓) |
[46] |
||||
|
Broccoli sprout powder |
Diabetes |
Anti-inflammatory effect |
Humans |
C-reactive protein (↓) |
[47] |
||||
|
Broccoli sprouts |
Hypertension |
Does not improve endothelial function of hypertension in humans |
Humans |
Not determined |
[48] |
( raphanistrum subsp. sativus) (7 days of growth) | |||
|
Broccoli sprouts |
Hypertension |
Attenuation of oxidative stress, hypertension, and inflammation |
Rats |
Kale (Brassica oleracea var. acephala) |
Flavonoids Quercetin and cyanidin |
Risk of cancer (↓) Heart disease (↓) Diabetes (↓) Antioxidant capacity (↑) |
[10] |
||
Dietary source of iodine | GPX1, GPX3, and FRAP (↓) |
[50] |
Phenolic acids Chlorogenic and ferulic acids |
||||||
|
Broccoli sprouts |
Hepatic and renal toxicity |
Antioxidant activity |
Female rats |
Glucosinolates Glucoraphanin, glucoiberin, gluconapin, gluconasturtin, progoitrin, gluconapin, gluconapoleiferin, sinigrin, glucobrassicin, 4-hydroxyglucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin |
|||||
Phase-II enzymes (↑) | Lipid peroxidation and apoptosis (↓) | [51] |
Pak choi (Brassica rapa | ||||||
|
Broccoli sprouts |
Bowel habits |
Decrease in the constipation scoring system Decrease of Bifidobacterium |
Humans |
Not determined |
[52] |
var. chinensis) |
Flavonoids Kaempferol, quercetin, and isorhamnetin glucosides |
Risk of cancer (↓) Heart disease (↓) Diabetes (↓) Antioxidant capacity (↑) |
|
] | |||||||||
|
Broccoli sprouts |
Pain assessment and analgesia |
Dose-dependent nociceptive activity |
Rats |
Agonists of central and peripheral opioid receptors |
[53] |
Phenolic acids Ferulic, sinapic, caffeic, and p-coumaric acids, and derivatives |
|||
|
Radish, Chinese kale and pak choi sprouts (3 days of growth) |
Glucose (5 g 100 mL−1) |
Biotic elicitor |
Hydroponic system for 3 days after sowing seeds |
Total phenolics (20.0%), gluconapin (150.0% and 60.0% in Chinese kale and pak choi, respectively), glucobrassicanapin (110-fold in pak choi) |
[33] |
Glucosinolates Gluconapin, glucoalyssin, gluconasturtin, progoitrin, glucobrassicin, 4-hydroxyglucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin |
3. Elicitation of Brassicaceae Sprouts to Enhance the Content of Bioactive (Poly)phenols and Glucosinolates
|
Raw Edible Sprout | |||||
|---|---|---|---|---|---|
Tuscan black cabbage sprout extract | |||||
Xenobiotic metabolism and antioxidant defense | |||||
Improvement of the detoxification of xenebiotics | Rats |
Induction of phase-II enzymes and boosting of the enzymatic activity of catalase, NAD(P)H:quinone reductase, glutathione reductase, and glutathione peroxidase |
[54] |
||
|
Different Brassica sprouts (broccoli, turnip, and rutabaga) |
MeJA (25 μM) JA (150 μM) Sucrose (146 mM) |
Biotic elicitors (Sucrose and plant hormones—MeJA and JA) |
Sprayed for 5 days before harvest |
Total GLS (>50%, broccoli; >20.0% turnip; >100.0% rutabaga) |
[34] |
|
Radish sprouts (raphanistrum subsp. sativus) (8 days of growth) |
MeJA (25 μM) SA (100 μM) Glucose (277 mM) |
Biotic elicitors (glucose and plant hormones—MeJA and JA) |
Sprayed for 5 days before harvest |
Total GLS (20.0%) |
[34] |
Japanese Radish Sprout | |||||
Diabetes | |||||
Decrease in plasma fructosamine, glucose, and insulin in diabetic rats | |||||
Rats | Not determined |
[40] |
|||
|
Radish sprouts |
Diabetes |
Increase in blood glucose, triglycerides, total cholesterol, low-density lipoproteins, and very low density lipoproteins |
Rats |
Not determined |
[55] |
|
Broccoli sprout extracts |
Skin disorders |
Induction of phase-II response |
Mice and humans |
NQO1 enzyme activity (↑) |
[56] |
|
Broccoli sprout extracts |
Skin disorders |
Protection against inflammation, edema, and carcinogens in humans |
Humans |
Phase-II enzymes (↑) NQO1 enzyme activity (↑) |
[57] |
|
Broccoli sprout homogenate |
Physiological upper airway |
No specific effect monitored |
Humans |
Phase-II enzymes (↑) |
[58] |
|
Broccoli sprouts |
Physiological upper airway |
No specific effect monitored |
Humans |
Nrf2 activity (↑) Secretory leukocyte protease inhibitor (↑) |
[59] |
|
Broccoli sprout extract |
Asthma |
Blocking the bronchoconstrictor hyperresponsiveness of some asthmatic phenotypes |
Humans |
Activity of Nrf2 regulated antioxidant and anti-inflammatory genes (↓) |
[60] |
|
Broccoli sprout extract |
Hepatic disturbances |
Improvement of liver functions and reduction of oxidative stress |
Rats |
Not determined |
[61] |
|
Broccoli sprout-based supplements |
General carcinogenic processes |
Chemopreventive effect |
Humans |
Not determined |
[62] |
|
Broccoli sprout extract |
Head and neck squamous cell carcinoma |
Chemopreventive activity of sulforaphane against carcinogen-induced oral cancer |
Mice |
Time and dose dependent induction of Nrf2 and Nrf2 target genes (NQO1 and GCLC) Dephosphorilation of pSTAT3 |
[63] |
|
Broccoli sprouts homogenate |
Sickle cell disease (hemoglobinopathy) |
Change in the gene expression levels |
Humans |
Expression of Nrf2 targets (HMOX1 and HBG1) (↑) |
[64] |
|
Broccoli sprouts |
Oxidative stress |
Improvement in cholesterol metabolism and decrease in oxidative stress |
Humans |
Not determined |
[65] |
|
Broccoli sprouts |
General carcinogenic processes |
Chemopreventive agent |
Humans |
Histone deacetylase activity (↓) |
[66] |
|
Broccoli sprouts |
Unspecific frame |
Not determined |
Humans |
Histone deacetylase activity (↓) |
[67] |
|
Broccoli sprouts |
Antimicrobial activity against Helicobacter pylori |
Reduction of Helicobacter pylori colonization in mice Enhancement of sequelae of Helicobacter pylori infection in mice and humans |
Mice and humans |
Not determined |
[68] |
|
Broccoli sprout extract |
Allergic response |
Broccoli sprouts reduce the impact of particulate pollution of allergic disease and asthma |
Humans |
Not determined |
[69] |
|
Broccoli sprout extract |
Prostate cancer |
Inconclusive |
Humans |
Not determined |
[70] |
|
Broccoli sprout and myrosinase-treated broccoli sprout extracts |
Chemoprevention of carcinogenesis processes |
Inconclusive |
Humans |
No dose response was observed for molecular targets |
[71] |
|
Broccoli sprout extract |
Psychiatric disorders |
Improvement of the cognitive function in patients affected by schizophrenia |
Humans |
Not determined |
[72] |
|
Broccoli sprout extract |
Type II diabetes |
Reduction of fasting blood glucose and glycated hemoglobin |
Mice |
(↑) Nuclear translocation of Nrf2 (↓) Glucose production and intolerance |
[73] |
|
Broccoli sprout extract |
Neurological disorder |
Inconclusive improvement of Autism symptoms |
Humans |
(↑) Gene transcription in multiple cell signaling pathways |
[74] |
|
Broccoli sprout homogenate |
Viral infections |
Enhancement of antiviral defense response |
Humans |
Modulation of natural killer cell activation Production of granzyme B by natural killer cells (↑) |
[75] |
Z FA, fatty acids; FRAP, ferric reducing activity of plasma; GCLC, glutamate-cysteine ligase catalytic subunit; GPX1, cytosolic glutathione peroxidase-1; GPX3, cytosolic glutathione peroxidase-3; HBG1, Hemoglobin subunit gamma 1; HMOX1, heme oxygenase (decycling) 1; IL-6, interleukina 6; NAD(P)H, nicotinamide adenine dinucleotide phosphate; NQO1, NAD(P)H:quinone oxidoreductase 1; TNF-α, tumor necrosis factor-alpha; Nrf2, nuclear factor erythroid 2–related factor 2; pSTAT3, signal transducer and activator of transcription-3; TSH, thyroid stimulating hormone. (↓↑) Non-significant variation, (↓) decrease, and (↑) increase.
Authors Cristina García-Viguera, Ángel Abellán, Raúl Domínguez-Perles, and Diego A. Moreno, as co-authors, would like to thanks the funding of this research by the "Fundación Seneca" - Murcia Regional Agency for Science and Technology (CARM), Project Reference N# 20855/PI/18.
