Low-grade inflammation (LGI) has been suggested to be involved in the development of chronic diseases. Healthy dietary patterns, such as the Mediterranean diet (MD), may decrease the markers of LGI. Healthy Nordic diet (HND) has many similarities with MD, but its effects on LGI are less well known. Both of these dietary patterns emphasize the abundant use of fruits and vegetables (and berries in HND), whole grain products, fish, and vegetable oil (canola oil in HND and olive oil in MD), but restrict the use of saturated fat and red and processed meat.
1. Introduction
Low-grade inflammation (LGI) has been linked with the pathogenesis of several chronic diseases, such as cardiovascular diseases, type 2 diabetes, certain cancers, and neurodegenerative diseases [
1,
2,
3,
4]. It is well known that human adipose tissue is not only a storage for excess energy, but it is also an active endocrine organ producing and releasing a number of bioactive compounds, adipokines, of which some are known to be pro-inflammatory (e.g., tumor necrosis factor alfa (TNF-α), Interleukins (IL) 6 and 8), and some anti-inflammatory (e.g., adiponectin) [
5,
6]. Besides adipose tissue, white blood cells (e.g., monocytes), macrophages, and the liver produce pro- and anti-inflammatory factors [
7,
8]. It has also been suggested that there is an interaction between the gut microbiota and the immune system, and changes in the gut microbiota may contribute to LGI by resulting in a deficient immune response and impaired tolerance to commensal microorganisms [
9,
10]. Various mechanisms through which LGI may contribute to the development of non-communicable diseases have been discussed in several reviews [
3,
6,
7,
11].
Obesity, especially abdominal obesity and the accumulation of visceral fat, is associated with LGI, and weight loss is the key treatment for LGI in overweight and obese people [
12]. Weight loss decreases the elevated concentrations of plasma inflammatory markers and increases e.g., low levels of anti-inflammatory adiponectin [
12,
13,
14,
15]. However, the quality of diet and its individual components may also affect the concentrations of inflammatory markers, regardless of weight loss [
12]. Especially the Mediterranean diet and some other healthy dietary patterns have been shown to decrease the markers of LGI [
16,
17]. Those dietary patterns have been characterized as plant food based ones (mostly fruit, vegetables and whole grain, and with a little red meat) [
16].
Healthy Nordic diet (HND) has many similarities with the Mediterranean diet [
18]. Both dietary patterns emphasize the abundant use of fruits and vegetables, whole grain products, and fish, but will restrict the use of saturated fat (milk fat) and red and processed meat. Olive oil is an important source for unsaturated fat in the Mediterranean diet, whereas canola oil is used in the HND. The HND includes also local berries, like bilberries, lingonberries, and strawberries [
18]. There is some variation in the HND between the different Nordic countries and regions, e.g., in consumed fish species and types of berries, fruits, vegetables, and bread.
Recently, Kaluza and colleagues developed a questionnaire-based Anti-Inflammatory Diet Index (AIDI), which could predict systemic chronic inflammation in the Nordic population [
19]. It was based on a 123-item food frequency questionnaire among 3503 women with high sensitivity C reactive protein (hsCRP) plasma concentration <20 mg/L. Altogether, 20 foods were statistically significantly related to hsCRP. Foods with anti-inflammatory potential were fruits and vegetables, tea, coffee, whole-grain bread, breakfast cereal, low-fat cheese, chocolate, dried fruits, herbal tea, olive and canola oils, legumes, nuts, linseeds, red wine, and beer. Foods with pro-inflammatory potential were unprocessed red meat, processed meat, organ meat, chips, and soft-drink beverages. This study did not investigate the complete dietary pattern, but hypothesized that these foods might be related to LGI, especially in the Nordic population.
2. HND and LGI in Observational Studies
Thus far, there are two observational studies (including data from three cohorts) investigating the association between the HND (named as the Baltic Sea Diet in these studies, which corresponds the same as HND) and LGI. Those studies have consistently shown an inverse association between the HND and hsCRP (
Table 2A).
The association between the HND and inflammatory markers was independently investigated in two large cross-sectional studies in Finnish participants: in the DIetary, Lifestyle, and Genetic determinants of Obesity and Metabolic syndrome (DILGOM) study (
n = 4579), and in the Helsinki Birth Cohort Study (HBCS,
n = 1911) [
23]. In both studies, the participants filled in a standardized and validated food frequency questionnaire (FFQ) that was designed to measure the habitual diet over the previous 12 months. Baltic Sea Dietary Score (BSDS), which reflects the HND (score from 0 to 25), was calculated based on the FFQ, and it included nine dietary features: (1) Nordic fruits (apples, pears, and berries); (2) Nordic vegetables (tomatoes, cucumber, leafy vegetables, roots, cabbages, peas); (3) Nordic cereals (rye, oat, and barley); (4) low-fat and fat-free milk; (5) Nordic fish (salmon and freshwater fish); (6) ratio of PUFA to SFA and trans-fatty acids; (7) low intake of red and processed meat; (8) total fat (as % of total energy); and, (9) moderate or low intake of alcohol. The higher the BSDS the better the adherence to the HND. LGI was measured using the following inflammatory markers: leptin, high-molecular weight (HMW) adiponectin, TNF-alfa, IL-6, and hsCRP. In the Determinants of Obesity and Metabolic Syndrome (DILGOM) study, hsCRP concentrations inversely associated with the BSDS (
p < 0.01) in all adjusted models, which included relevant confounding factors, such as age, sex, energy intake, education, smoking, physical activity, waist circumference, medication for diabetes, and the use of statins. Participants’ mean concentration of hsCRP was 1.14 mg/L in the lowest BSDS quintile and 1.03 mg/L in the highest BSDS quintile. Unexpectedly, HMW-adiponectin concentrations also inversely associated with the BSDS (
p < 0.05). Adjustments for the above-mentioned confounding factors strengthened this association (
p < 0.01). Other measured inflammatory markers did not associate with BSDS in the DILGOM study. Alcohol intake was the only component that significantly associated with the HMW-adiponectin concentrations of the single BSDS components. Participants with a high intake of alcohol had higher HMW-adiponectin concentration than individuals with low alcohol intake (
p < 0.001). This finding is consistent with earlier findings that were related to alcohol consumption and adiponectin [
31]. In the HBCS, the hsCRP inversely associated with the BSDS in all adjusted models (
p < 0.01). Other measured inflammatory markers did not associate with BSDS in the HBCS study. Among the single BSDS components, the higher intake of Nordic fruits, berries, and cereals, and the lower intake of red and processed meat and moderate alcohol intake significantly associated with lower hsCRP concentrations in both of the studies.
Both the DILGOM and the HBCS study were included among the Health 2000 Survey in the meta-analysis investigating associations with BSDS and cardiometabolic risk factors, including hsCRP [
22] (
Table 2A). In this meta-analysis, the risk of elevated hsCRP concentration was lower both among men (OR 0.58, 95% CI 0.43, 0.78) and women (OR 0.73, 95% CI 0.58, 0.91) in the highest BSDS quintile than among those in the lowest BSDS quintile.
In summary, it has been consistently shown that a high adherence to the HND lowers the risk of low-grade inflammation, but, so far, the studies are scarce and only conducted among Finns.
3. Nordic Diet and LGI in Randomized Dietary Trials
3.1. Studies Including Selected Key Components of the HND
Only three intervention studies have investigated the effects of the HND pattern on LGI (
Table 2B). Furthermore, the effect of the selected key components of the HND on LGI has been studied in several randomized dietary trials. For example, bilberries, which are rich in phenolic compounds [
32] and commonly used in the Nordic countries, decreased the inflammatory score, which was calculated based on concentrations of hsCRP, IL-6, IL-12 and LPS, during the 8-week intervention period [
33]. We investigated the effect of low insulin response grain products (including rye bread, sourdough whole grain wheat bread, and dark pasta), fatty fish, and bilberries on several inflammatory markers in the SYSDIMET intervention [
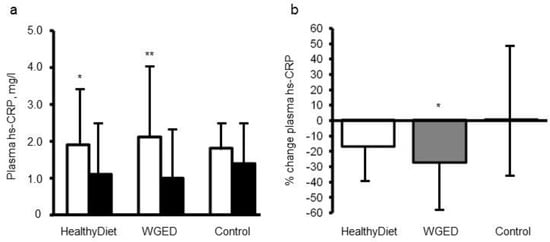
29]. Altogether, 104 participants with characteristics of metabolic syndrome who had completed the study were analyzed. The study participants were randomized for 12 weeks into one of three dietary groups: (1) a Healthy Diet group (included whole grain and low insulin response grain products, fish and bilberries), (2) Whole Grain Enriched Diet group (WGED), and (3) control diet group (included refined cereal products and limited fish and berry consumption). HsCRP, TNF-α, IL-6, IL1Ra serum amyloid A (SAA), chemokine (C-C motif) ligand 5 (CCL5), soluble intercellular cellular adhesion molecule-1 (sICAM-1), and macrophage migration inhibitory factor (MIF) were measured as markers of LGI before and after the intervention. There were no significant differences in the changes in these inflammatory markers, except in hsCRP in the participants, who did not use statins during the intervention. The plasma hsCRP concentrations decreased in individuals following the WGED and Healthy Diet interventions (
p < 0.01 and
p < 0.05, respectively) and the change in hsCRP in the WGED group was significantly different from that in the control group (
p < 0.05) (
Figure 1). Interestingly, after the intervention, hsCRP was at the same level as in statin users, who had low levels already in the onset of the study (
Figure 1).
Figure 1. Plasma concentrations (
a) and relative changes (
b) of high sensitivity C-reactive protein (hsCRP) according to study group in the participants not using statins during the SYSDIMET intervention [
29]. (
a) White bars represent hsCRP concentrations at baseline and black bars after a 12 week consumption of HND (
n = 27), Whole Grain Enriched Diet (WGED) (
n = 24) or control diet (
n = 25).*
p < 0.05 and **
p < 0.001 for baseline vs. week 12 in Student’s paired test. (
b)
p = 0.04 for the group effect in general linear model univariate analysis. *
p < 0.05 for the difference between WGED and control groups after Bonferroni correction for multiple comparisons. Values are median and interquartile range (IQR).
The effect of a prudent low-fat breakfast (based on Nordic foods) as compared with usual breakfast was studied in a parallel controlled 12-week study with 79 healthy overweight [
34]. The prudent breakfast included oat bran porridge with low-fat milk or yogurt, bilberry or lingonberry jam, whole grain bread, low-fat spread (high in PUFA), poultry or fatty fish, and fruit or berries. Foods were provided
ad libitum. CRP and TNF-R2 was significantly decreased by the prudent breakfast when compared with the control breakfast (
p < 0.005). The percentual changes in CRP and TNF-R2 between baseline and 12 weeks were +37% for the control breakfast and −30% for the prudent breakfast, and +9% for the control breakfast and +2% for the prudent breakfast, respectively. There were no changes in body weight, but sagittal abdominal diameter, which is a marker of visceral fat, was reduced in the prudent breakfast group.
3.2. HND in Controlled Dietary Trials
The effect of the HND pattern on LGI has been investigated in three randomized controlled trials (
Table 2B). In the Dietary Intervention With Shop Model (SHOPUS), the objective was to investigate whether the
ad libitum New Nordic Diet (NND) versus Average Danish Diet (ADD) in adults (18–65 y) with central obesity and components of the metabolic syndrome could reduce body weight and improve the risk markers of the metabolic syndrome, type 2 diabetes, and cardiovascular diseases [
25,
28]. The NND included foods as fruits and vegetables (especially berries, cabbages, root vegetables, and legumes), potatoes, fresh herbs, wild plants, and mushrooms, nuts, whole grain, meats from livestock and game, fish and shellfish, and seaweed [
35]. The control ADD was the diet that was typically eaten by the adult Danish population. The decrease in energy intake and weight loss was greater in the NND group as compared with the ADD group. The plasma CRP concentration decreased in the NND group (
p = 0.007) [
27]. The decrease of CRP attenuated, but remained significant after adjusting for weight loss (
p = 0.043). TNF-α was measured for the subset of the study population (
n = 64), and it did not change significantly [
25]. It is worth knowing that these beneficial results were not sustained in the follow-up of the study [
36]. However, higher compliance with NND after the active intervention was associated with less body weight regain [
36].
In the NORDIET trial, 88 normal to slightly overweight hyperlipidemic men and women were randomly assigned to
ad libitum Nordic prudent diet or a control (habitual) diet for six weeks [
26,
27]. Altogether, 86 participants completed the study with measurements of circulating CRP and cathepsin S levels. Similarly as in the SHOPUS study, the body weight decreased in the HND group as compared with the controls, despite the
ad libitum nature of the HND diet. Cathepsin S is a proteolytic enzyme that has been associated with LGI [
37,
38]. Compared with a habitual control diet, the HND decreased cathepsin S levels (
p = 0.03). However, the decrease was not significant after adjusting for change in body weight and LDL-cholesterol concentration, which may indicate that the decrease of the level of S cathepsin was mediated by weight loss or lowered LDL cholesterol concentration. There were no changes in CRP (
p = 0.40) [
26].
In the SYSDIET study [
24], altogether 200 middle-aged individuals with characteristics of metabolic syndrome and impaired fasting glucose/glucose intolerance were randomized into a HND group or a control group for 18 to 24 weeks in six study centers in Finland (2), Sweden (2), Denmark (1), and Iceland (1). The diets were isocaloric in order to keep body weight unchanged. HND included whole-grain products, local berries, fruits and vegetables, canola oil, three fish meals per week and low-fat dairy products. Compliance to diet was monitored by repeated food diaries and serum phospholipid fatty acid profile [
24], and later on by alcylresorcinols (fiber intake) and serum beta-carotene (intake of vegetables) [
39]. There were more drop-outs in the control group (27% vs. 7.9%), and 96 subjects in the HND group and 70 subjects in the control group completed the study. Some differences in responses to dietary changes were found across different study centers, but, there was an improved lipid profile with HND when compared to control group, and lipid changes and blood pressure reduction were more beneficial in most compliant participants of the HND group when examined in more detail in relation to compliance. No significant changes were found in the weight or glucose tolerance between the study groups. Among the various inflammatory markers examined (hsCRP, IL-1beta, IL-1Ra, IL-6, IL-10, and TNF RII, HMW adiponectin), only IL-1Ra showed a different response between the diets: it increased in the control group while it remained stable in the HND, and at the end of the study a marked difference was seen in IL-1Ra between the groups. The high intake of saturated fatty acids associated with an increase in IL-1Ra, whereas magnesium intake was inversely related to IL-1Ra, but the association with dietary fiber intake was not significant [
24]. These results on IL-1Ra may be of importance, since IL-1Ra is a sensitive marker of insulin resistance and it has been shown to predict type 2 diabetes [
40]. HND tended to increase plasmalogens based on the further analyses of the serum samples from the SYSDIET study, while a decreasing trend was found in ceramides in line with anti-oxidative and anti-inflammatory properties of HND [
41]. Furthermore, in two sub-studies of the SYSDIET, down-regulations of immune response related genes were reported in adipose tissue [
20] and PBMCs [
30]. Recently, we found associations between dietary fiber intake, serum indole propionate coming from gut, inflammation, and insulin secretion capacity in the study participants of the Finnish Diabetes Prevention Study followed over 10 years. This suggests an intimate role of dietary fiber in the regulation of glucose metabolism and LGI [
42,
43].
-
Summary
Quite few studies have investigated the effect of HND on LGI so far. Two observational studies have shown inverse association between the adherence to the HND and concentration of hsCRP. In the intervention studies, significant decrease in the concentration of hsCRP has been reported in two studies [28,29] out of four studies measuring hsCRP [24,26,28,29]. Furthermore, the HND has had beneficial effects on concentrations of other inflammatory markers such as IL-1Ra [24] and Cathepsin S [27] and it has been shown to downregulate gene expression of inflammation related genes in adipose tissue and PBMC [20,30]. It is noteworthy that these positive effects have been seen without significant weight loss. These results suggest that the HND may have anti-inflammatory effects, but more carefully controlled studies are needed to confirm the anti-inflammatory effects of the HND.