You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Giovanni Casini.
Diabetic retinopathy (DR) is one of the most common complications of diabetes mellitus and is characterized by degeneration of retinal neurons and neoangiogenesis, causing a severe threat to vision. Nowadays, the principal treatment options for DR are laser photocoagulation, vitreoretinal surgery, or intravitreal injection of drugs targeting vascular endothelial growth factor. Treatment with nutraceuticals (foods providing medical or health benefits) at early stages of DR may represent a reasonable alternative to act upstream of the disease, preventing its progression.
- retina
- oxidative stress
- inflammation
- microvascular lesions
- neoangiogenesis
- polyphenols
- flavonoids
- carotenoids
- saponins
1. Introduction
Diabetic retinopathy (DR) is a retinal disease representing one of the main causes of vision loss in developed countries. It has been classically considered a microvascular disease of the retina and is characterized, in its later stages, by abnormal growth of retinal vessels, which causes hemorrhages and tractional retinal detachment, leading to vision loss[1]. The understanding of DR has evolved over time and has clarified the role of the neuronal component of the retina in the progression of the disease. Indeed, growing experimental evidence suggests that suffering and death of retinal neurons occur before overt vascular changes[2][3][4]. For this reason, nowadays DR can be described not only as a microvascular but also as a neurodegenerative disease of the retina[5].
DR is a multifactorial disease but, to date, the exact pathophysiological mechanisms underlying neuro-vascular damage are not thoroughly understood. Nevertheless, different pathways and molecular mechanisms that may cause DR onset have been studied. For instance, the increase in advanced glycation end-products (AGEs) acting at their receptors (RAGE), the formation and activation of protein kinase C (PKC), or the increased flux in the polyol or hexosamine pathway have been examined[6][7]. All of these pathways, along with lower levels of glutathione (GSH), are associated with an increase in oxidative stress. The latter in turn causes different alterations in the diabetic retina as a consequence of severe lipid peroxidation, protein oxidation, oxidative DNA damage, induction of inflammation, and upregulation of growth factors, such as vascular endothelial growth factor (VEGF)[8].
VEGF is a proangiogenic factor that plays a key role in the late vasculopathy. For this reason, current DR treatments consist of the intraocular delivery of anti-VEGF molecules whose action induces restriction or inhibition of abnormal vessel growth. Nevertheless, the administration of anti-VEGF drugs has limitations and may generate different side effects. In addition, the effects are not long-lasting and frequent intravitreal injections are necessary[9][10][11].
Recent studies have highlighted the neuroprotective role of VEGF that can be noticed in the early phases of DR[12][13]. According to these studies, retinal neurons stressed by diabetes are likely to trigger the release of VEGF as a survival strategy. However, the persistence of the upstream stress conditions determines the accumulation of VEGF, leading to disruption of the blood-retina barrier (BRB) and, in the long term, to neoangiogenesis[14]. It would therefore be appropriate to plan new therapeutic strategies acting upstream of the disease and to prevent its progression by reducing neuronal stress and favoring neuroprotection. Moreover, considering the side effects caused by therapeutic agents administered via intraocular injections, there is a need to develop compounds with antioxidant and/or anti-inflammatory activity that can be administered through alternative delivery modalities. For this reason, in the last few years, several studies have focused on the potential benefits of nutraceuticals.
The term “nutraceutical” was coined by Dr. Stephen De Felice in 1989 and indicates “a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of a disease”[15]. Nutraceuticals are effective antioxidants. They may induce the expression of antioxidant enzymes, act as scavengers of reactive oxygen species (ROS), or display singlet oxygen-quenching activity, as in the case of carotenoids[16]. Nutraceuticals may also exert anti-inflammatory effects by reducing the expression or nuclear translocation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)[17]. Nutraceuticals can be used as natural dietary supplements and therefore can be easily administered, are readily available, and are affordable. A further advantage of nutraceuticals is that they are not likely to induce collateral side effects (if, of course, delivered at the appropriate dosage) such as hypoglycemia, liver injury, or gastric complains, which are characteristic of well-known and popular drugs[18][19] (Figure 1).
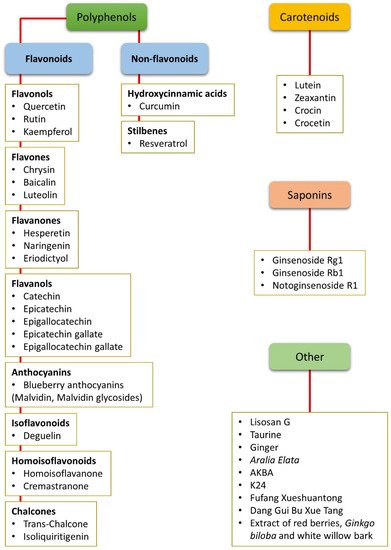
Figure 1. Summary of all the nutraceuticals cited in the present review. The compounds are listed according to their chemical classes, including polyphenols (both flavonoids and non-flavonoids), carotenoids, and saponins. Other compounds that do not belong to any of these classes or that are mixtures of different chemicals are classified as “other”. AKBA: Acetyl-11-keto-β-boswellic acid.
2. Nutraceuticals and Oxidative Stress
Oxidative stress is caused by an imbalance in the production of ROS and the activity of the biological detoxifying systems. ROS are produced in normal metabolic conditions to support normal cellular functions and modulate a variety of biological processes including cell proliferation, differentiation, and migration, signal transduction, and programmed cell death[8]. However, because of ROS’ high reactivity, their accumulation compromises the cell structure and functionality through alterations and degradation of molecules such as DNA, lipids, and proteins[20]. Oxidative stress and ROS production are contrasted by endogenous antioxidant defense enzymes including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-P), and glutathione reductase (GSH-R). In addition to these endogenous enzymatic systems, endogenous non-enzymatic factors also exist and they include GSH (which is regulated by GSH-P and GSH-R), vitamin C, and vitamin E[21]. Besides endogenous antioxidant defenses, exogenous antioxidants of natural origin may be used to preserve redox homeostasis. They may act directly as scavengers of free radicals, indirectly by interrupting free radical chain reactions, or both. They may also decrease oxidative stress by inducing the expression of endogenous antioxidant enzymes[22][23]. For these reasons, it has been recently proposed that therapies based on natural, non-enzymatic antioxidants such as nutraceuticals could relieve the decrease in endogenous antioxidant defenses[23][24].
The retina is highly susceptible to oxidative stress, which is due principally to the high content of polyunsaturated fatty acids, high oxygen uptake, glucose oxidation, and prolonged exposure to light. In particular, high glucose levels trigger a set of processes, such as AGE accumulation, PKC activation, and increased flux in the polyol and hexosamine pathways, which provoke oxidative stress (see[25] for detail). In turn, an increase in ROS is likely to cause DNA fragmentation resulting in poly-ADP ribose polymerase activation and glyceraldehyde 3-phosphate dehydrogenase inhibition[26]. This causes accumulation of glycolytic metabolites that may induce AGE formation and activation of PKC and of the polyol as well as of the hexosamine pathways, which are known to contribute to DR pathogenesis[6][7]. In summary, oxidative stress creates a propagating cycle, causing a continuous increase in ROS and consequent activation of pathways closely related to the progression of DR[8] (Figure 2).
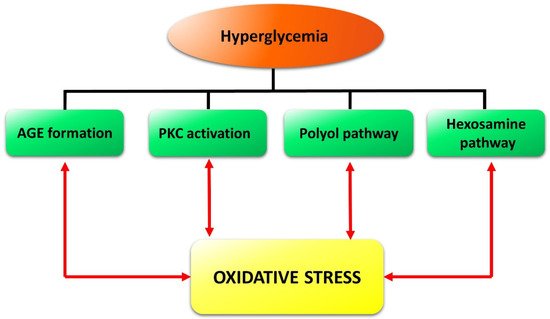
Figure 2. Schematic reconstruction of the events triggered in the retina by hyperglycemia and reinforced by oxidative stress in a vicious cycle. Formation of advanced glycation end-products (AGE) as well as the activation of protein kinase C (PKC), of the polyol pathway, and of the hexosamine pathway, are the main diabetes-induced abnormalities related to diabetic retinopathy.
Different natural dietary compounds have been investigated as possible treatments or adjuvants to counteract retinal oxidative stress typical of DR. They include polyphenols, carotenoids, and saponins, as well as other compounds (Figure 1). They are common in different fruits, vegetables, herbs, and beverages, and are very efficient in strengthening the endogenous antioxidant defenses through a direct scavenger activity and/or through the stimulation of antioxidant enzyme expression. Several classes of these compounds have been tested in vitro and in in vivo animal models. A summary of the effects of nutraceuticals against oxidative stress in models of DR is given in Figure 3.
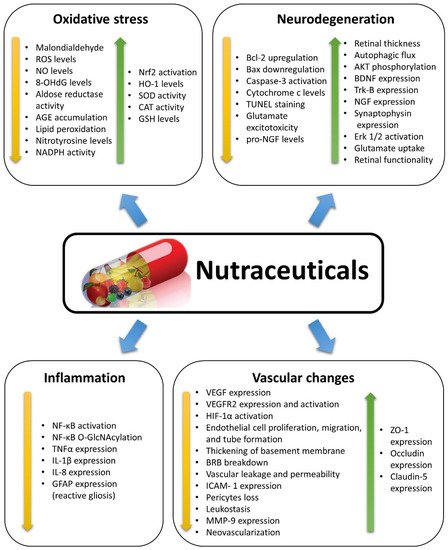
Figure 3. Summary of the effects induced by nutraceuticals as described in the studies reviewed herein. Nutraceuticals exert positive effects in diabetic retinopathy, counteracting the diabetes-induced changes by decreasing (yellow arrows) or increasing (green arrows) the expression/activation of specific factors or the occurrence of some events. 8-OHdG: 8-hydroxy-2′-deoxyguanosine; AGE: Advanced glycation end-products; AKT: Protein kinase B; Bax: Bcl-2-associated X protein; Bcl-2: B cell lymphoma 2; BDNF: Brain-derived neurotrophic factor; BRB: Blood-retina barrier; CAT: Catalase; Erk 1/2: Extracellular signal-regulated kinase 1/2; GFAP: Glial fibrillary acidic protein; GSH: Glutathione; HIF-1α: Hypoxia inducible factor 1α; HO-1: Heme oxygenase-1; ICAM-1: Intercellular cell adhesion molecule 1; IL-1β: Interleukin 1 beta; MMP-9: Matrix metalloproteinase-9; NADPH: Nicotinamide adenine dinucleotide phosphate; Nf-kB: Nuclear factor kappa-light-chain-enhancer of activated B cells; O-GlcNAc: O-linked β-N-acetylglucosamine; NGF: Nerve growth factor; NO: Nitric oxide; Nrf2: Transcription nuclear factor erythroid-2-related factor-2; ROS: Reactive oxygen species; SOD: Superoxide dismutase; TNFα: tumor necrosis factor alpha; Trk-B: Tyrosine receptor kinase B; TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling; VEGF: Vascular endothelial growth factor; VEGFR2: Vascular endothelial growth factor receptor 2; ZO-1: Zonula occludens 1.
3. Nutraceuticals and Inflammation
Inflammation is a nonspecific response to injury that includes a variety of functional mediators, such as cytokines, chemokines, acute phase proteins, and other pro-inflammatory molecules. Many of these mediators have been detected in the retina of diabetic animals or patients, suggesting that inflammation has a role in the development of DR[27][28][29]. Reactive gliosis, characterized by increased glial fibrillary acidic protein (GFAP) expression in both Müller cells and astrocytes[4][30], is typically observed in DR[31][32], resulting in the release from these cells of inflammatory cytokines, such as tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL-1β), and others[33][34].
The transcription of inflammatory proteins is regulated by the activation of pro-inflammatory transcription factors, among which NF-κB plays a prominent role. This factor, once activated, translocates into the nucleus, binds to nuclear DNA, and acts as a master switch that promotes the expression of pro-inflammatory cytokines such as IL-1β, interleukin 6, interleukin 8 (IL-8), and, at least in part, TNFα[35]. There is ample evidence suggesting that NF-κB is involved in the pathogenesis of the early phases of DR. In fact, the inhibition of proteins whose expression is regulated by NF-κB decreases capillary degeneration, while direct NF-κB blockade inhibits DR development and progression[27][28][29][36]. The potential efficacy of some nutraceuticals for the treatment of DR is that they may inhibit NF-κB activation. A summary of the effects of nutraceuticals against inflammation in models of DR is given in Figure 3.
4. Nutraceuticals and Neurodegeneration
DR is characterized by an extended loss of neurons due to an increase in apoptosis likely paralleled by a decrease in autophagic capabilities[37]. Neuronal cell vulnerability is evident very early in DR, and it is detectable before any sign of vascular damage[2][3][4]. This early neuronal impairment leads to retinal functional deficits that can be recorded with electroretinography (ERG) and that are associated with different morphological changes, these mostly including a decrease in thickness of retinal layers, with INL and IPL affected in particular. In retinas of diabetic rodents, an increase in terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) positive cells can be recorded together with a decrease in anti-apoptotic markers (e.g., B cell lymphoma 2 (Bcl-2)) and an increase in pro-apoptotic markers (e.g., active caspase-3 and Bcl-2-associated X protein (Bax))[38][39][40]. Neurodegeneration in DR is likely caused by high glucose-induced oxidative stress and inflammation, but there is evidence that dysregulation of neurotrophic factor expression may also play a role. Neurotrophin nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) are expressed by retinal neurons and glia, and are principally involved in cell survival and synaptic modulation[41][42]. A reduction in neurotrophin expression or an imbalance between the mature neurotrophin and its precursor (as in the case of proNGF/NGF) may lead to neuronal damage and neurodegeneration[42][43]. A further cause of neuronal death in DR is represented by increased glutamate levels causing excitotoxicity. This condition is likely to be due to oxidative stress in Müller cells resulting in decreased activity of glutamate-aspartate transporters and down-regulation of glutamine synthetase (GS), which converts glutamate into non-toxic glutamine[44]. Several natural compounds are known for their neuroprotective properties and for their positive effects within the central nervous system. In particular, nutraceuticals rich in flavonoids have been proposed for the treatment and prevention of a variety of neurodegenerative diseases[45][46]. A summary of the effects of nutraceuticals against neurodegeneration in models of DR is given in Figure 3.5. Nutraceuticals and Vascular Changes
On the basis of vascular changes, DR is classified as a non-proliferative diabetic retinopathy (NPDR) or proliferative diabetic retinopathy (PDR). NPDR is characterized by microvascular damage including BRB breakdown, pericyte loss, acellular capillaries, capillary occlusion, and thickening of the basement membrane. In PDR, neoangiogenesis phenomena are observed and new blood vessels are generated. These vessels create a deleterious action in the retina because of their mechanic traction, which, in the end, causes retinal detachment and consequent blindness[47]. As outlined below, VEGF, acting at its main receptor vascular endothelial growth factor receptor-2 (VEGFR2), plays prominent roles in both phases of DR. The BRB represents a filter allowing selective passage of substances from the bloodstream to the retina, thereby regulating osmotic equilibrium, ionic concentrations, and transport of nutrients. These functions are based on the presence of tight and adherens junctions between adjacent cells. Tight junctions are composed of proteins like occludin, claudin, and zonula occludens 1 (ZO-1). These proteins are the principal compounds implicated in BRB functionality, creating a strong bond between endothelial cells and regulating the transport of solutes and molecules through prevention of the unchecked diffusion of substances between the bloodstream and neuroretina[48]. In DR, oxidative stress and inflammation result in complex changes causing upregulation of cytokines and growth factors, among which VEGF is the most implicated in BRB dysfunctions[49][50]. Indeed, VEGF upregulation is correlated with alterations of the tight junction structure caused by VEGF-induced phosphorylation and downregulation of tight junction proteins (i.e., ZO-1 and occludin)[51][52]. In addition, overexpressed VEGF also induces phosphorylation of the adherens junction protein VE-cadherin, further favoring increased BRB permeability[53]. VEGF upregulation in DR also correlates with increased expression of intercellular cell adhesion molecule 1 (ICAM-1), which in turn promotes leucocyte adhesion and capillary occlusion[13]. Other cytokines and chemokines are implicated in BRB impairment. For instance, TNFα overexpression is associated with decreases in occludin, claudin, and ZO-1 expression, while IL-1β induces barrier dysfunction through leukocyte recruitment and release of the vasoactive amine histamine[54][55]. Matrix metalloproteinases (MMPs) play important roles both in the early stages of DR, when MMP-2 and MMP-9 promote the apoptosis of retinal capillary cells, and in the later phase, when they facilitate neovascularization by degrading the extracellular matrix[56]. Other early vascular pathological changes in NPDR include loss of pericytes and thickening of the basement membrane. Pericytes are contractile cells located at the surface of capillaries, implicated in blood vessel stability, blood flow regulation, and formation of the BRB. In NPDR, pericyte loss occurs even before endothelial injury and is directly correlated with accumulation of AGEs, impairment of the BRB, and vascular leakage[57][58]. Apoptosis of pericytes in NPDR also leads to formation of microaneurysms and acellular capillaries[59]. Thickening of the basement membrane, due to the increase in vascular basal membrane compounds such as laminin and collagen IV[50], may contribute to the disruption of the tight link between pericytes and endothelial cells, causing pericyte apoptosis, whereas the endothelium, deprived of proliferation control, can give rise to new vessels[60]. PDR is characterized by neovascularization coupled with fibrotic responses at the vitreoretinal interface, and subsequent blindness due to vitreous hemorrhage, retinal fibrosis, tractional retinal detachment, and neovascular glaucoma[61][62][63]. Out of all the angiogenesis regulators, VEGF has been most extensively studied and provides the basis for current anti-angiogenic therapy[64]. VEGF plays a crucial role in PDR pathogenesis by promoting neovascularization through binding to VEGFR2 expressed on endothelial cells, inducing endothelial cell proliferation and sprouting angiogenesis[65]. The protective actions of nutraceuticals against microvascular changes typical of NPDR have been investigated in a variety of DR models. However, these models do not reproduce the neoangiogenesis characterizing PDR, and evidence of possible antiangiogenic properties of nutraceuticals has been found in other experimental models favoring the growth of new retinal vessels, mainly rodents with oxygen induced retinopathy (OIR) or experimental choroidal neovascularization (CNV). Other indications of the possible antiangiogenic effects of nutraceuticals have been derived from observations of their efficacy in inhibiting endothelial cell proliferation, migration, and tube formation. A summary of the effects of nutraceuticals against vascular changes in models of DR or of neoangiogenesis is given in Figure 3.6. Bioavailability of Nutraceuticals
Bioavailability is a pharmacokinetic term referring to the fraction of bioactive compound that reaches the blood circulation without undergoing alterations. The index of bioavailability of nutraceuticals is important because it allows for the calculation of the right dose of nutraceutical to ingest. For this reason, understanding the oral bioavailability of a nutraceutical compound is as important as understanding its therapeutic potential. After ingestion, botanical compounds must overcome a series of threats that may alter their structure before they can reach systemic circulation, for instance, the environment of the gastrointestinal tract and the intestinal as well as the hepatic metabolism. Unfortunately, many nutraceuticals have low oral bioavailability, and therefore investigations to improve this aspect are of fundamental importance. Recently, significant steps forward have been made to develop new technologies using analogous compounds, nanoformulations, or nanoparticles, which may protect the nutraceutical from enteric adverse conditions[66][67][68][69].
Curcumin is characterized by poor bioavailability mainly due to low solubility, rapid metabolism and poor absorption, which, despite its medical efficacy, limits its clinical applications[70]. Conjugation of curcumin to metal oxide nanoparticles or encapsulation in lipid nanoparticles, dendrimers, nanogels, or polymeric nanoparticles, improves the water solubility and bioavailability of curcumin, thus increasing its pharmacological effectiveness[71]. The encapsulation of curcumin in the calix[4] arene nanoassembly limits curcumin degradation and increases its solubility, enhancing the effect of the compound on antioxidant and anti-inflammatory markers in both in vivo and in vitro models[72]. Similar results have been obtained using a different nanocarrier formulation comprising Pluronic-F127 stabilized d-α-Tocopherol polyethene glycol 1000 succinate nanoparticles[73]. A recent study has reported that, among different tested curcumin formulations, only that containing a hydrophilic carrier may provide therapeutic levels of curcumin in rabbit retinas[74].
Resveratrol, similarly to curcumin, is known for its poor oral bioavailability and scarce pharmacokinetic properties due to low aqueous solubility and low photostability, which compromise its great potential. In fact, as shown by pharmacokinetic studies, the levels of unmetabolized resveratrol after oral administration are reduced to about 1% due to its high intestinal and hepatic metabolism[75]. To solve this problem, different resveratrol nanoformulations have been tested, including liposomes, solid lipid nanoparticles, polymeric nanoparticles, and cyclodextrins. The use of these alternative administration methods generates different advantages because they improve solubility, bioavailability, and physical chemical stability, and favor a controlled drug release[76][77][78]. The use of resveratrol analogs could be another alternative choice for administration of this nutraceutical. The pharmacokinetic profiles of resveratrol and its analog perolstilbene have been analyzed in rats, showing that the bioavailability of perolstilbene was 80% and that of resveratrol 20%[79]. A summary of oral delivery systems for resveratrol has recently been published[80].
Nanoparticles can also be used to increase the bioavailability of epigallocatechin gallate, another nutraceutical characterized by low solubility and stability. Different nanosystems have been used for epigallocatechin gallate delivery, including liposomes, gold nanoparticles, inorganic nanocarriers, and lipid as well as polymeric nanoparticles[81][82].
A recent study has reported that the distribution of an orally administered nutraceutical may vary substantially depending on tissue type. Indeed, in a pilot study, 13C-lutein was detected in a variety of tissues in a rhesus macaque after a single oral administration, but not in the retina[83]. Some improvement in lutein delivery to ocular tissues may derive from lutein encapsulation into hyaluronic acid-coated PLGA nanoparticles, which have been demonstrated to efficiently bind ARPE-19 cells and improve the physicochemical properties of lutein[84].
References
- Curtis, T.; Gardiner, T.; Stitt, A. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye 2009, 23, 1496, doi: 10.1038/eye.2009.108.
- Harrison, W.W.; Bearse, M.A.; Ng, J.S.; Jewell, N.P.; Barez, S.; Burger, D.; Schneck, M.E.; Adams, A.J. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investigative ophthalmology & visual science 2011, 52, 772-777, doi: 10.1167/iovs.10-5931.
- Hernández, C.; Dal Monte, M.; Simó, R.; Casini, G. Neuroprotection as a therapeutic target for diabetic retinopathy. Journal of diabetes research 2016, 2016, doi: 10.1155/2016/9508541.
- Simo, R.; Hernandez, C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends in Endocrinology & Metabolism 2014, 25, 23-33, doi: 10.1016/j.tem.2013.09.005.
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia 2018, 1-11, doi: 10.1007/s00125-018-4692-1.
- arr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of diabetic retinopathy. ISRN ophthalmology 2013, 2013, doi: 10.1155/2013/343560.
- Wang, W.; Lo, A. Diabetic retinopathy: pathophysiology and treatments. International journal of molecular sciences 2018, 19, 1816, doi: 10.3390/ijms19061816.
- Kowluru, R.A.; Chan, P.-S. Oxidative stress and diabetic retinopathy. Experimental diabetes research 2007, 2007, doi:10.1155/2007/43603.
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI insight 2017, 2, doi: 10.1172/jci.insight.93751.
- Simó, R.; Sundstrom, J.M.; Antonetti, D.A. Ocular anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes care 2014, 37, 893-899. doi: 10.2337/dc13-2002.
- Zhao, Y.; Singh, R.P. The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs in context 2018, 7, doi: 10.7573/dic.212532.
- Amato, R.; Biagioni, M.; Cammalleri, M.; Dal Monte, M.; Casini, G. VEGF as a survival factor in ex vivo models of early diabetic retinopathy. Investigative ophthalmology & visual science 2016, 57, 3066-3076, doi: 10.1167/iovs.16-19285.
- Behl, T.; Kotwani, A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacological research 2015, 99, 137-148, doi: 10.1016/j.phrs.2015.05.013.
- Witmer, A.; Vrensen, G.; Van Noorden, C.; Schlingemann, R. Vascular endothelial growth factors and angiogenesis in eye disease. Progress in retinal and eye research 2003, 22, 1-29, doi:10.1016/S1350-9462(02)00043-5.
- Brower, V. Nutraceuticals: poised for a healthy slice of the healthcare market? Nature biotechnology 1998, 16, 728, doi:10.1038/nbt0898-728.
- Milatovic, D.; Zaja-Milatovic, S.; Gupta, R.C. Oxidative Stress and Excitotoxicity: Antioxidants from Nutraceuticals. In Nutraceuticals, Elsevier: 2016; pp. 401-413, doi:10.3390/molecules15117792.
- Aggarwal, B.B.; Van Kuiken, M.E.; Iyer, L.H.; Harikumar, K.B.; Sung, B. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Experimental Biology and Medicine 2009, 234, 825-849, doi: 10.3181/0902-MR-78.
- Chauhan, B.; Kumar, G.; Kalam, N.; Ansari, S.H. Current concepts and prospects of herbal nutraceutical: a review. Journal of advanced pharmaceutical technology & research 2013, 4, 4, doi: 10.4103/2231-4040.107494.
- Kalra, E.K. Nutraceutical-definition and introduction. Aaps Pharmsci 2003, 5, 27-28, doi: 10.1208/ps050325.
- Calderon, G.; Juarez, O.; Hernandez, G.; Punzo, S.; De la Cruz, Z. Oxidative stress and diabetic retinopathy: development and treatment. Eye 2017, 31, 1122, doi: 10.1038/eye.2017.64.
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461-482, doi: 10.1007/s10522-013-9463-2.
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51, doi: 10.3390/antiox6030051.
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Advances 2015, 5, 27986-28006, doi: 10.1039/C4RA13315C.
- Cui, K.; Luo, X.; Xu, K.; Murthy, M.V. Role of oxidative stress in neurodegeneration: recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2004, 28, 771-799, doi: 10.1016/j.pnpbp.2004.05.023.
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid Med Cell Longev 2018, 5, doi: 10.1155/2018/3420187.
- Brownlee, M. The pathobiology of diabetic complications: a unifying mechanism. diabetes 2005, 54, 1615-1625, doi.org/10.2337/diabetes.54.6.1615.
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B. A central role for inflammation in the pathogenesis of diabetic retinopathy. The FASEB journal 2004, 18, 1450-1452, doi: 10.1096/fj.03-1476fje.
- Kern, T.S. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Journal of Diabetes Research 2007, 2007, doi: 10.1155/2007/95103.
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Progress in retinal and eye research 2011, 30, 343-358, doi: 10.1016/j.preteyeres.2011.05.002.
- Lieth, E.; Gardner, T.W.; Barber, A.J.; Antonetti, D.A. Retinal neurodegeneration: early pathology in diabetes. Clinical & Experimental Ophthalmology: Viewpoint 2000, 28, 3-8, doi.org/10.1046/j.1442-9071.2000.00222.x.
- Lieth, E.; Barber, A.J.; Xu, B.; Dice, C.; Ratz, M.J.; Tanase, D.; Strother, J.M. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes 1998, 47, 815-820, doi.org/10.2337/diabetes.47.5.815.
- Mizutani, M.; Gerhardinger, C.; Lorenzi, M. Muller cell changes in human diabetic retinopathy. Diabetes 1998, 47, 445-449, doi.org/10.2337/diabetes.47.3.445.
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res 2009, 28, 423-451, doi: 10.1016/j.preteyeres.2009.07.001.
- Lee, H.J.; Suk, J.E.; Patrick, C.; Bae, E.J.; Cho, J.H.; Rho, S.; Hwang, D.; Masliah, E.; Lee, S.J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 2010, 285, 9262-9272, doi: 10.1074/jbc.M109.081125.
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2017, 2, 14, doi: 10.1038/sigtrans.2017.23.
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-kappabeta: A Potential Target in the Management of Vascular Complications of Diabetes. Front Pharmacol 2017, 8, doi: 10.3389/fphar.2017.00798.
- Amato, R.; Catalani, E.; Dal Monte, M.; Cammalleri, M.; Di Renzo, I.; Perrotta, C.; Cervia, D.; Casini, G. Autophagy-mediated neuroprotection induced by octreotide in an ex vivo model of early diabetic retinopathy. Pharmacol Res 2018, 128, 167-178, doi: 10.1016/j.phrs.2017.09.022.
- Barber, A.J. Diabetic retinopathy: recent advances towards understanding neurodegeneration and vision loss. Sci China Life Sci 2015, 58, 541-549, doi: 10.1007/s11427-015-4856-x.
- Barber, A.J.; Baccouche, B. Neurodegeneration in diabetic retinopathy: Potential for novel therapies. Vision Res 2017, 139, 82-92, doi: 10.1016/j.visres.2017.06.014.
- Ola, M.S.; Nawaz, M.I.; Khan, H.A.; Alhomida, A.S. Neurodegeneration and neuroprotection in diabetic retinopathy. Int J Mol Sci 2013, 14, 2559-2572, doi: 10.3390/ijms14022559.
- Ola, M.S.; Alhomida, A.S. Neurodegeneration in diabetic retina and its potential drug targets. Curr Neuropharmacol 2014, 12, 380-386, doi: 10.2174/1570159X12666140619205024.
- Mohamed, R.; El-Remessy, A.B. Imbalance of the Nerve Growth Factor and Its Precursor: Implication in Diabetic Retinopathy. J Clin Exp Ophthalmol 2015, 6, 2155-9570, doi: 10.4172/2155-9570.1000483.
- Ola, M.S.; Nawaz, M.I.; El-Asrar, A.A.; Abouammoh, M.; Alhomida, A.S. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell Mol Neurobiol 2013, 33, 359-367, doi: 10.1007/s10571-012-9901-8.
- Li, Q.; Puro, D.G. Diabetes-induced dysfunction of the glutamate transporter in retinal Muller cells. Invest Ophthalmol Vis Sci 2002, 43, 3109-3116.
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv Nutr 2015, 6, 64-72, doi: 10.3945/an.114.007500.
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr 2008, 3, 115-126, doi: 10.1007/s12263-008-0091-4.
- Stitt, A.W.; Lois, N.; Medina, R.J.; Adamson, P.; Curtis, T.M. Advances in our understanding of diabetic retinopathy. Clinical Science 2013, 125, 1-17, doi:10.1042/Cs20120588.
- Frey, T.; Antonetti, D.A. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal 2011, 15, 1271-1284, doi: 10.1089/ars.2011.3906.
- Eshaq, R.S.; Aldalati, A.M.Z.; Alexander, J.S.; Harris, N.R. Diabetic retinopathy: Breaking the barrier. Pathophysiology 2017, 24, 229-241, doi: 10.1016/j.pathophys.2017.07.001.
- Kusuhara, S.; Fukushima, Y.; Ogura, S.; Inoue, N.; Uemura, A. Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes & Metabolism Journal 2018, 42, 364-376, doi:10.4093/dmj.2018.0182, doi: 10.4093/dmj.2018.0182.
- Antonetti, D.A.; Barber, A.J.; Hollinger, L.A.; Wolpert, E.B.; Gardner, T.W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem 1999, 274, 23463-23467, doi: 10.1074/jbc.274.33.23463.
- Murakami, T.; Felinski, E.A.; Antonetti, D.A. Occludin Phosphorylation and Ubiquitination Regulate Tight Junction Trafficking and Vascular Endothelial Growth Factor-induced Permeability. Journal of Biological Chemistry 2009, 284, 21036-21046, doi:10.1074/jbc.M109.016766.
- Esser, S.; Lampugnani, M.G.; Corada, M.; Dejana, E.; Risau, W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. Journal of Cell Science 1998, 111, 1853-1865.
- Aveleira, C.A.; Lin, C.M.; Abcouwer, S.F.; Ambrosio, A.F.; Antonetti, D.A. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 2010, 59, 2872-2882, doi: 10.2337/db09-1606.
- Bamforth, S.D.; Lightman, S.L.; Greenwood, J. Interleukin-1 beta-induced disruption of the retinal vascular barrier of the central nervous system is mediated through leukocyte recruitment and histamine. American Journal of Pathology 1997, 150, 329-340.
- Kowluru, R.A.; Zhong, Q.; Santos, J.M. Matrix metalloproteinases in diabetic retinopathy: potential role of MMP-9. Expert Opinion on Investigational Drugs 2012, 21, 797-805, doi:10.1517/13543784.2012.681043.
- Hammes, H.P.; Lin, J.H.; Renner, O.; Shani, M.; Lundqvist, A.; Betsholtz, C.; Brownlee, M.; Deutsch, U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 2002, 51, 3107-3112, doi: 10.2337/diabetes.51.10.3107.
- Jing-Xu; Chen, L.J.; Yu, J.; Wang, H.J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cellular Physiology and Biochemistry 2018, 48, 705-717, doi:10.1159/000491897.
- Ejaz, S. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obesity & Metabolism 2008, 10, 53-63, doi:10.1111/j.1463-1326.2007.00795.x.
- Beltramo, E.; Porta, M. Pericyte Loss in Diabetic Retinopathy: Mechanisms and Consequences. Current Medicinal Chemistry 2013, 20, 3218-3225, doi: 10.2174/09298673113209990022.
- Loukovaara, S.; Gucciardo, E.; Repo, P.; Vihinen, H.; Lohi, J.; Jokitalo, E.; Salven, P.; Lehti, K. Indications of lymphatic endothelial differentiation and endothelial progenitor cell activation in the pathology of proliferative diabetic retinopathy. Acta Ophthalmologica 2015, 93, 512-523, doi:10.1111/aos.12741.
- Roy, S.; Amin, S.; Roy, S. Retinal fibrosis in diabetic retinopathy. Experimental Eye Research 2016, 142, 71-75, doi:10.1016/j.exer.2015.04.004.
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R., et al. The progress in understanding and treatment of diabetic retinopathy. Progress in Retinal and Eye Research 2016, 51, 156-186, doi:10.1016/j.preteyeres.2015.08.001.
- Adamis, A.P.; Miller, J.W.; Bernal, M.T.; Damico, D.J.; Folkman, J.; Yeo, T.K.; Yeo, K.T. Increased Vascular Endothelial Growth-Factor Levels in the Vitreous of Eyes with Proliferative Diabetic-Retinopathy. American Journal of Ophthalmology 1994, 118, 445-450, doi:10.1016/S0002-9394(14)75794-0.
- Osaadon, P.; Fagan, X.J.; Lifshitz, T.; Levy, J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye 2014, 28, 510-520, doi:10.1038/eye.2014.13.
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Current Opinion in Colloid & Interface Science 2009, 14, 3-15, doi:10.1016/j.cocis.2008.01.002.
- Huang, Q.R.; Yu, H.L.; Ru, Q.M. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. Journal of Food Science 2010, 75, R50-R57, doi:10.1111/j.1750-3841.2009.01457.x.
- Ting, Y.W.; Jiang, Y.; Ho, C.T.; Huang, Q.R. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. Journal of Functional Foods 2014, 7, 112-128, doi:10.1016/j.jff.2013.12.010.
- Yao, M.F.; McClements, D.J.; Xiao, H. Improving oral bioavailability of nutraceuticals by engineered nanoparticle-based delivery systems. Current Opinion in Food Science 2015, 2, 14-19, doi:10.1016/j.cofs.2014.12.005.
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Molecular Pharmaceutics 2007, 4, 807-818, doi:10.1021/mp700113r.
- Shome, S.; Talukdar, A.D.; Choudhury, M.D.; Bhattacharya, M.K.; Upadhyaya, H. Curcumin as potential therapeutic natural product: a nanobiotechnological perspective. J Pharm Pharmacol 2016, 68, 1481-1500, doi: 10.1111/jphp.12611.
- Granata, G.; Paterniti, I.; Geraci, C.; Cunsolo, F.; Esposito, E.; Cordaro, M.; Blanco, A.R.; Cuzzocrea, S.; Consoli, G.M.L. Potential Eye Drop Based on a Calix[4]arene Nanoassembly for Curcumin Delivery: Enhanced Drug Solubility, Stability, and Anti Inflammatory Effect. Molecular Pharmaceutics 2017, 14, 1610-1622, doi:10.1021/acs.molpharmaceut.6b01066.
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H., et al. Topical Curcumin Nanocarriers are Neuroprotective in Eye Disease. Scientific Reports 2018, 8, doi:10.1038/S41598-018-29393-8.
- Platania, C.B.M.; Fidilio, A.; Lazzara, F.; Piazza, C.; Geraci, F.; Giurdanella, G.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. Retinal Protection and Distribution of Curcumin in Vitro and in Vivo. Front Pharmacol 2018, 9, doi: 10.3389/fphar.2018.00670.
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacological Research 2012, 66, 375-382, doi:10.1016/j.phrs.2012.08.001.
- Bonechi, C.; Martini, S.; Ciani, L.; Lamponi, S.; Rebmann, H.; Rossi, C.; Ristori, S. Using Liposomes as Carriers for Polyphenolic Compounds: The Case of Trans-Resveratrol. Plos One 2012, 7, doi:10.1371/journal.pone.0041438.
- Jung, K.H.; Lee, J.H.; Park, J.W.; Quach, C.H.T.; Moon, S.H.; Cho, Y.S.; Lee, K.H. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. International Journal of Pharmaceutics 2015, 478, 251-257, doi:10.1016/j.ijpharm.2014.11.049.
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. International Journal of Pharmaceutics 2015, 479, 282-290, doi:10.1016/j.ijpharm.2015.01.003.
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.H.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemotherapy and Pharmacology 2011, 68, 593-601, doi:10.1007/s00280-010-1525-4.
- Popescu, M.; Bogdan, C.; Pintea, A.; Rugina, D.; Ionescu, C. Antiangiogenic cytokines as potential new therapeutic targets for resveratrol in diabetic retinopathy. Drug Des Devel Ther 2018, 12, 1985-1996, doi: 10.2147/DDDT.S156941.
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. Biomed Res Int 2017, 5813793, 16, doi: 10.1155/2017/5813793.
- Minnelli, C.; Moretti, P.; Fulgenzi, G.; Mariani, P.; Laudadio, E.; Armeni, T.; Galeazzi, R.; Mobbili, G. A Poloxamer-407 modified liposome encapsulating epigallocatechin-3-gallate in the presence of magnesium: Characterization and protective effect against oxidative damage. Int J Pharm 2018, 552, 225-234, doi: 10.1016/j.ijpharm.2018.10.004.
- Jeon, S.; Li, Q.; Rubakhin, S.S.; Sweedler, J.V.; Smith, J.W.; Neuringer, M.; Kuchan, M.; Erdman, J.W., Jr. (13)C-lutein is differentially distributed in tissues of an adult female rhesus macaque following a single oral administration: a pilot study. Nutr Res 2019, 61, 102-108. doi: 10.1016/j.nutres.2018.10.007.
- Chittasupho, C.; Posritong, P.; Ariyawong, P. Stability, Cytotoxicity, and Retinal Pigment Epithelial Cell Binding of Hyaluronic Acid-Coated PLGA Nanoparticles Encapsulating Lutein. AAPS PharmSciTech 2018, 20, 018-1256, doi: 10.1208/s12249-018-1256-0.
More
