It explores the renewable ways of obtaining methanol and its use in efficient energy systems for a net zero-emission carbon cycle, with a special focus on fuel cells. It investigates the different parts of the carbon cycle from a methanol and fuel cell perspective. In recent years, the potential for a methanol economy has been shown and there has been significant technological advancement of its renewable production and utilization. Even though its full adoption will require further development, it can be produced from renewable electricity and biomass or CO2 capture and can be used in several industrial sectors, which make it an excellent liquid electrofuel for the transition to a sustainable economy. By converting CO2 into liquid fuels, the harmful effects of CO2 emissions from existing industries that still rely on fossil fuels are reduced. The methanol can then be used both in the energy sector and the chemical industry, and become an all-around substitute for petroleum.
- methanol
- electrofuels
- power-to-X
- high temperature PEM
- fuel cell
- reforming
1. Introduction
2 em
ission from electricity production to a record low 150 gCO2/kWhel in 2019 from 199 gCO2/kWhel in 2018 [8]. The drop is even more striking when compared to the emission levels of 2010 of 426 gCO2/kWhel CO2/kWhel
[8].
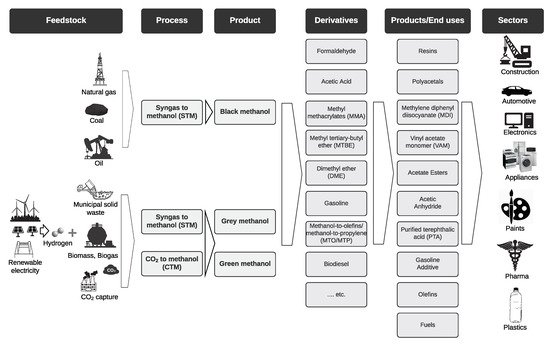
2. Methanol Production
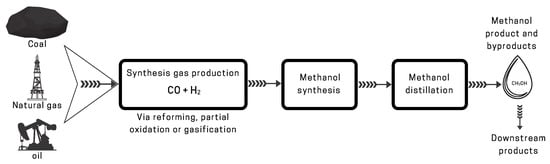
2.1. Traditional Methods
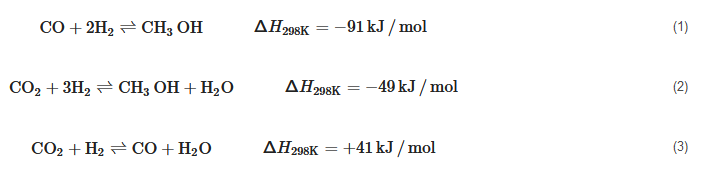
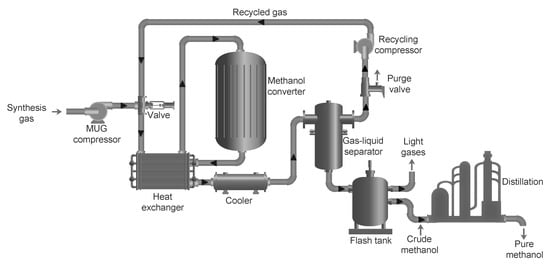
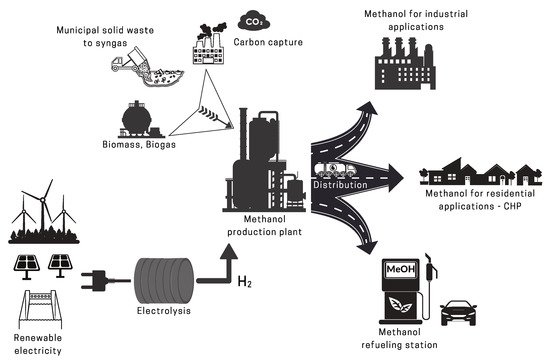
2.2. Renewable Methanol
| Step | Energy Conversion Route | Process Efficiency | Reference | ||
|---|---|---|---|---|---|
| Methanol | Hydrogen | Methanol | Hydrogen | ||
| 1 | Power Management, Conditioning and Transmission | 90% | [190] | ||
| 2 | H2 production by electrolysis | PEMEC → 78% | [191] | ||
| AEC → 74% | [192] | ||||
| SOEC → 96% | [71] | ||||
| 3 | Methanol synthesis | H2 compression | 79 (69–89)% | 75 (65–85)% | [83] |
| 4 | Fuel transportation | 99% | 95% | [193] | |
| 5 | Methanol Fuel cell system | Hydrogen Fuel cell system | 50% | 60% | [145,194] |
2.2.1. Technology Status and Prospects
2.2.2. Renewable CO2 Sources
2.2.3. Renewable H2 Sources

3. Methanol Use
3.1. Methanol in The Chemical Industry
3.2. Methanol in Energy Systems
3.2.1. Methanol in Internal Combustion Engines
3.2.2. Methanol in Fuel Cells
Direct Methanol Fuel Cells (DMFC)
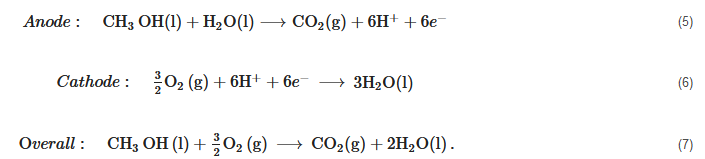
High Temperature PEM Fuel Cells (HT-PEMFC)
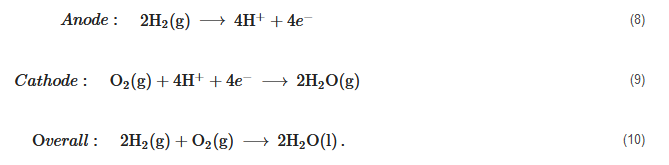
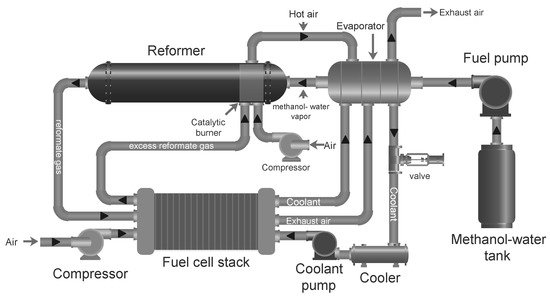
Methanol Reforming
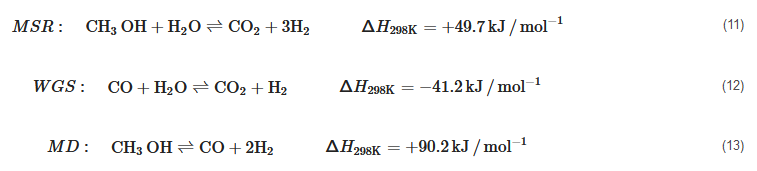
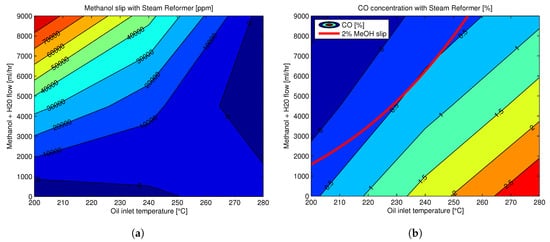
HT-PEMFC Technology Status and Prospects
References
- Shindell, D.; Smith, C.J. Climate and air-quality benefits of a realistic phase-out of fossil fuels. Nature 2019, 573, 408–411.
- Smith, C.J.; Forster, P.M.; Allen, M.; Fuglestvedt, J.; Millar, R.J.; Rogelj, J.; Zickfeld, K. Current fossil fuel infrastructure does not yet commit us to 1.5 °C warming. Nat. Commun. 2019, 10.
- Shindell, D.; Faluvegi, G.; Seltzer, K.; Shindell, C. Quantified, localized health benefits of accelerated carbon dioxide emissions reductions. Nat. Clim. Chang. 2018, 1–5.
- Weindl, I.; Lotze-Campen, H.; Popp, A.; Müller, C.; Havlík, P.; Herrero, M.; Schmitz, C.; Rolinski, S. Livestock in a changing climate: production system transitions as an adaptation strategy for agriculture. Environ. Res. Lett. 2015, 10, 094021.
- Lelieveld, J.; Klingmüller, K.; Pozzer, A.; Burnett, R.T.; Haines, A.; Ramanathan, V. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl. Acad. Sci. USA 2019, 116, 7192–7197.
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918.
- IEA. Global Energy & CO2 Status Report 2019. Available online: https://www.iea.org/reports/global-energy-and-co2-status-report-2019/emissions (accessed on 17 January 2020).
- ENERGINET. Rekordlav CO2-udledning fra danskernes elforbrug i 2019. Available online: https://energinet.dk/Om-nyheder/Nyheder/2020/01/16/Rekord-lav-CO2udledning-fra-danskernes-elforbrug-i-2019 (accessed on 17 January 2020).
- Frensch, S.H.; Olesen, A.C.; Simon Araya, S.; Kær, S.K. Model-Supported Analysis of Degradation Phenomena of a PEM Water Electrolysis Cell under Dynamic Operation. ECS Trans. 2018, 85, 37–45.
- Larscheid, P.; Lück, L.; Moser, A. Potential of new business models for grid integrated water electrolysis. Renew. Energy 2018, 125, 599–608.
- Goldmann, A.; Sauter, W.; Oettinger, M.; Kluge, T.; Schröder, U.; Seume, J.R.; Friedrichs, J.; Dinkelacker, F. A study on electrofuels in aviation. Energies 2018, 11, 392.
- Hobson, C.; Márquez, C. Renewable Methanol Report; Technical report; ATA Markets Intelligence S.L. on behalf of the Methanol Institute: Madrid, Spain, 2018.
- Goeppert, A.; Czaun, M.; Surya Prakash, G.K.; Olah, G.A. Air as the renewable carbon source of the future: An overview of CO2 capture from the atmosphere. Energy Environ. Sci. 2012, 5, 7833–7853.
- Brown, A.; Le Feuvre, P. Technology Roadmap: Delivering Sustainable Bioenergy; International Energy Agency (IEA): Paris, France, 2017; p. 94.
- Cui, X.; Kær, S.K. Thermodynamic analyses of a moderate-temperature carbon dioxide hydrogenation to methanol via reverse water gas shift process with in situ water removal. Ind. Eng. Chem. Res. 2019.
- Alberico, E.; Nielsen, M. Towards a methanol economy based on homogeneous catalysis: Methanol to H2 and CO2 to methanol. Chem. Commun. 2015, 51, 6714–6725.
- Methanol Institute. Methanol|Methanol Institute. Available online: https://www.methanol.org (accessed on 10 December 2019).
- Zhao, K. A Brief Review of China’s Methanol Vehicle Pilot and Policy; Technical report; Methanol Institute: Alexandria, VA, USA, 2019.
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy; Wiley-VCH: Hoboken, NJ, USA, 2006.
- Olah, G.A. Towards Oil Independence Through Renewable Methanol Chemistry. Angew. Chem. Int. Ed. 2013, 52, 104–107.
- Offermanns, H.; Schulz, K.; Brandes, E.; Schendler, T. Methanol Utilisation Technologies. In Methanol: The Basic Chemical and Energy Feedstock of the Future: Asinger’s Vision Today; Bertau, M., Offermanns, H., Plass, L., Schmidt, F., Wernicke, H.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 327–601.
- Ott, J.; Gronemann, V.; Pontzen, F.; Fiedler, E.; Grossmann, G.; Kersebohm, D.B.; Weiss, G.; Witte, C. Methanol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012.
- El-Zeftawy, A.M. Focus on the Chemical Value of Methanol. J. Univ. Eng. Sci. 1995, 7, 209–254.
- Khadzhiev, S.N.; Kolesnichenko, N.V.; Ezhova, N.N. Manufacturing of lower olefins from natural gas through methanol and its derivatives (review). Pet. Chem. 2008, 48, 325–334.
- Zhen, X.; Wang, Y. An overview of methanol as an internal combustion engine fuel. Renew. Sustain. Energy Rev. 2015, 52, 477–493.
- Dalena, F.; Senatore, A.; Basile, M.; Knani, S.; Basile, A.; Iulianelli, A. Advances in Methanol Production and Utilization, with Particular Emphasis toward Hydrogen Generation via Membrane Reactor Technology. Membranes 2018, 8, 98.
- Alvarado, M. The Changing Face of the Global Methanol Industry; Technical report; IHS: London, UK, 2016.
- Market Watch. Methanol Market 2019 Analysis and Technological Innovation by Leading Key Players 2026. Available online: https://www.marketwatch.com/press-release/methanol-market-2019-analysis-and-technological-innovation-by-leading-key-players-2026-2019-08-07 (accessed on 18 January 2020).
- Sheldon, D. Methanol Production - A Technical History. Johns. Matthey Technol. Rev. 2017, 61, 172–182.
- Bertau, M.; Offermanns, H.; Plass, L.; Schmidt, F.; Wernicke, H.J. Methanol: The Basic Chemical and Energy Feedstock of the Future: Asinger’s Vision Today; Springer: Berlin/Heidelberg, Germany, 2014.
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Chapter 1—Methanol Production and Applications: An Overview. In Methanol; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–28.
- Wernicke, H.J.; Plass, L.; Schmidt, F. Methanol Generation. In Methanol: The Basic Chemical and Energy Feedstock of the Future: Asinger’s Vision Today; Bertau, M., Offermanns, H., Plass, L., Schmidt, F., Wernicke, H.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 51–301.
- Rostrup-Nielsen, J.; Christiansen, L.J. Concepts in Syngas Manufacture. In Catalyst Science Series; Imerial Collage Press: London, UK, 2011; Volume 10, p. 379.
- Cui, X.; Kær, S.K. Two-dimensional thermal analysis of radial heat transfer of monoliths in small-scale steam methane reforming. Int. J. Hydrog. Energy 2018, 43, 11952–11968.
- Lange, J.P. Methanol synthesis: a short review of technology improvements. Catal. Today 2001, 64, 3–8.
- Bell, D.; Towler, B.; Fan, M. Coal Gasification and Its Applications, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; p. 416.
- Liu, K.; Song, C.; Subramani, V. Hydrogen and Syngas Production and Purification Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–533.
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105.
- Barbieri, G.; Marigliano, G.; Golemme, G.; Drioli, E. Simulation of CO2 hydrogenation with CH3OH removal in a zeolite membrane reactor. Chem. Eng. J. 2002, 85, 53–59.
- Riaz, A.; Zahedi, G.; Klemeš, J.J. A review of cleaner production methods for the manufacture of methanol. J. Clean. Prod. 2013, 57, 19–37.
- Palma, V.; Meloni, E.; Ruocco, C.; Martino, M.; Ricca, A. State of the Art of Conventional Reactors for Methanol Production. In Methanol Science and Engineering; Angelo, B., Francesco, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 29–51.
- Blug, M.; Leker, J.; Plass, L.; Günther, A. Methanol Generation Economics. In Methanol: The Basic Chemical and Energy Feedstock of the Future: Asinger’s Vision Today; Bertau, M., Offermanns, H., Plass, L., Schmidt, F., Wernicke, H.J., Eds.; Springer: Berlin, Germany, 2014; pp. 603–618.
- Dahl, P.J.; Ostergaard, J. Process for Production of DME from Crude Methanol. U.S. Patent 10,011,548, 3 July 2018.
- Isayama, Y.; Saka, S. Biodiesel production by supercritical process with crude bio-methanol prepared by wood gasification. Bioresour. Technol. 2008, 99, 4775–4779.
- Zhang, J.; Liang, S.; Feng, X. A novel multi-effect methanol distillation process. Chem. Eng. Process. Process. Intensif. 2010, 49, 1031–1037.
- Cui, C.; Xi, Z.; Liu, S.; Sun, J. An enumeration-based synthesis framework for multi-effect distillation processes. Chem. Eng. Res. Des. 2019, 144, 216–227.
- Cui, C.; Sun, J.; Li, X. A hybrid design combining double-effect thermal integration and heat pump to the methanol distillation process for improving energy efficiency. Chem. Eng. Process. Process. Intensif. 2017, 119, 81–92.
- Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrog. Energy 2013, 38, 2039–2061.
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176.
- Serenergy A/S. Methanol Production. Available online: http://www.serenergy.com (accessed on 10 December 2019).
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Process. Process. Intensif. 2004, 43, 1029–1036.
- Dehghani, Z.; Bayat, M.; Rahimpour, M. Sorption-enhanced methanol synthesis: Dynamic modeling and optimization. J. Taiwan Inst. Chem. Eng. 2014, 45, 1490–1500.
- Arora, A.; Iyer, S.S.; Bajaj, I.; Faruque Hasan, M.M. Optimal Methanol Production via Sorption-Enhanced Reaction Process. Ind. Eng. Chem. Res. 2018, 57, 14143–14161.
- Bos, M.; Brilman, D. A novel condensation reactor for efficient CO2 to methanol conversion for storage of renewable electric energy. Chem. Eng. J. 2015, 278, 527–532.
- Bukhtiyarova, M.; Lunkenbein, T.; Kähler, K.; Schlögl, R. Methanol Synthesis from Industrial CO2 Sources: A Contribution to Chemical Energy Conversion. Catal. Lett. 2017, 147, 416–427.
- Liu, X.M.; Lu, G.Q.; Yan, Z.F.; Beltramini, J. Recent Advances in Catalysts for Methanol Synthesis via Hydrogenation of CO and CO2. Ind. Eng. Chem. Res. 2003, 42, 6518–6530.
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81.
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257.
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567.
- Dang, S.; Yang, H.; Gao, P.; Wang, H.; Li, X.; Wei, W.; Sun, Y. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today 2019, 330, 61–75.
- Roy, S.; Cherevotan, A.; Peter, S.C. Thermochemical CO2 Hydrogenation to Single Carbon Products: Scientific and Technological Challenges. ACS Energy Lett. 2018, 3, 1938–1966.
- Marlin, D.S.; Sarron, E.; Sigurbjörnsson, Ó. Process Advantages of Direct CO2 to Methanol Synthesis. Front. Chem. 2018, 6, 1–8.
- Pontzen, F.; Liebner, W.; Gronemann, V.; Rothaemel, M.; Ahlers, B. CO2-based methanol and DME - Efficient technologies for industrial scale production. Catal. Today 2011, 171, 242–250.
- Joo, O.S.; Jung, K.D.; Moon, I.; Rozovskii, A.Y.; Lin, G.I.; Han, S.H.; Uhm, S.J. Carbon dioxide hydrogenation to form methanol via a reverse-water-gas- shift reaction (the CAMERE process). Ind. Eng. Chem. Res. 1999, 38, 1808–1812.
- Joo, O.S.; Jung, K.D.; Yonsoo, J. CAMERE Process for methanol synthesis from CO2 hydrogenation. Stud. Surf. Sci. Catal. 2004, 153, 67–72.
- Anicic, B.; Trop, P.; Goricanec, D. Comparison between two methods of methanol production from carbon dioxide. Energy 2014, 77, 279–289.
- Samimi, F.; Rahimpour, M.R. Direct Methanol Fuel Cell. In Methanol Science and Engineering; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 381–397.
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463.
- Graves, C.; Ebbesen, S.D.; Mogensen, M. Co-electrolysis of CO2 and H2O in solid oxide cells: Performance and durability. Solid State Ionics 2011, 192, 398–403.
- Andika, R.; Nandiyanto, A.B.D.; Putra, Z.A.; Bilad, M.R.; Kim, Y.; Yun, C.M.; Lee, M. Co-electrolysis for power-to-methanol applications. Renew. Sustain. Energy Rev. 2018, 95, 227–241.
- Jensen, S.H.; Graves, C.; Chen, M.; Hansen, J.B.; Sun, X. Characterization of a planar solid oxide cell stack operated at elevated pressure. J. Electrochem. Soc. 2016, 163, F1596–F1604.
- Al-Kalbani, H.; Xuan, J.; García, S.; Wang, H. Comparative energetic assessment of methanol production from CO2: Chemical versus electrochemical process. Appl. Energy 2016, 165, 1–13.
- Sun, X.; Chen, M.; Jensen, S.H.; Ebbesen, S.D.; Graves, C.; Mogensen, M. Thermodynamic analysis of synthetic hydrocarbon fuel production in pressurized solid oxide electrolysis cells. Int. J. Hydrog. Energy 2012, 37, 17101–17110.
- Ebbesen, S.D.; Hansen, J.B.; Mogensen, M.B. Biogas upgrading using SOEC with a Ni-ScYSZ electrode. In ECS Transactions; Electrochemical Society Inc.: Pennington, NJ, USA, 2013; Volume 57, pp. 3217–3227.
- Yoneima, T.; Fukushima, K.; Saito, N.; Nakashima, K. Effect of Sulfur on the Sintering of Nickel Particles. Mater. Trans. 2016, 57, 1374–1377.
- Brett, D.J.L.; Atkinson, A.; Cumming, D.; Ramírez-Cabrera, E.; Rudkin, R.; Brandon, N.P. Methanol as a direct fuel in intermediate temperature (500–600 °C) solid oxide fuel cells with copper based anodes. Chem. Eng. Sci. 2005, 60, 5649–5662.
- Skafte, T.L.; Guan, Z.; Machala, M.L.; Gopal, C.B.; Monti, M.; Martinez, L.; Stamate, E.; Sanna, S.; Garrido Torres, J.A.; Crumlin, E.J.; et al. Selective high-temperature CO2 electrolysis enabled by oxidized carbon intermediates. Nat. Energy 2019, 4, 846–855.
- Saito, M.; Takeuchi, M.; Fujitani, T.; Toyir, J.; Luo, S.; Wu, J.; Mabuse, H.; Ushikoshi, K.; Mori, K.; Watanabe, T. Advances in joint research between NIRE and RITE for developing a novel technology for methanol synthesis from CO2 and H2. Appl. Organomet. Chem. 2000, 14, 763–772.
- Toyir, J.; Miloua, R.; Elkadri, N.; Nawdali, M.; Toufik, H.; Miloua, F.; Saito, M. Sustainable process for the production of methanol from CO2 and H2 using Cu/ZnO-based multicomponent catalyst. Phys. Procedia 2009, 2, 1075–1079.
- Doss, B.; Ramos, C.; Atkins, S. Optimization of Methanol Synthesis from Carbon Dioxide and Hydrogen: Demonstration of a Pilot-Scale Carbon-Neutral Synthetic Fuels Process. Energy Fuels 2009, 23, 4647–4650.
- Deerberg, G.; Oles, M.; Schlögl, R. The Project Carbon2Chem®. Chemie-Ingenieur-Technik 2018, 90, 1365–1368.
- Power2Met. Available online: https://energiforskning.dk/en/node/9313 (accessed on 10 December 2019).
- Brynolf, S.; Taljegard, M.; Grahn, M.; Hansson, J. Electrofuels for the transport sector: A review of production costs. Renew. Sustain. Energy Rev. 2018, 81, 1887–1905.
- Klenert, D.; Mattauch, L.; Combet, E.; Edenhofer, O.; Hepburn, C.; Rafaty, R.; Stern, N. Making carbon pricing work for citizens. Nat. Clim. Chang. 2018, 8, 669–677.
- Ramstein, C.; Dominioni, G.; Ettehad, S.; Lam, L.; Quant, M.; Zhang, J.; Mark, L.; Nierop, S.; Berg, T.; Leuschner, P.; et al. State and Trends of Carbon Pricing 2019; The World Bank: Washington, DC, USA, 2019.
- Laude, A.; Ricci, O.; Bureau, G.; Royer-Adnot, J.; Fabbri, A. CO2 capture and storage from a bioethanol plant: Carbon and energy footprint and economic assessment. Int. J. Greenh. Gas Control. 2011, 5, 1220–1231.
- Lin, W.C.; Chen, Y.P.; Tseng, C.P. Pilot-scale chemical–biological system for efficient H2S removal from biogas. Bioresour. Technol. 2013, 135, 283–291.
- Bao, Z.; Yu, F. Catalytic Conversion of Biogas to Syngas via Dry Reforming Process. Adv. Bioenergy 2018, 3, 43–76.
- Kumar, N.; Shojaee, M.; Spivey, J.J. Catalytic bi-reforming of methane: From greenhouse gases to syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15.
- Hansen, J.B.; Fock, F.; Lindboe, H.H. Biogas upgrading: By steam electrolysis or co-electrolysis of biogas and steam? ECS Trans. 2013, 57, 3089–3097.
- International Energy Agency (IEA). Technology Roadmap: Carbon Capture and Storage; Technical report; IEA: Paris, France, 2013.
- Odeh, N.A.; Cockerill, T.T. Life cycle GHG assessment of fossil fuel power plants with carbon capture and storage. Energy Policy 2008, 36, 367–380.
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443.
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732.
- Holloway, S. Safety of the underground disposal of carbon dioxide. Energy Convers. Manag. 1997, 38, S241–S245.
- Ehteshami, S.M.M.; Chan, S.H. The role of hydrogen and fuel cells to store renewable energy in the future energy network - potentials and challenges. Energy Policy 2014, 73, 103–109.
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Solar Energy 2005, 78, 661–669.
- Kiaee, M.; Cruden, A.; Infield, D.; Chladek, P. Improvement of power system frequency stability using alkaline electrolysis plants. Proc. Inst. Mech. Eng. Part J. Power Energy 2013, 227, 115–123.
- Guinot, B.; Montignac, F.; Champel, B.; Vannucci, D. Profitability of an electrolysis based hydrogen production plant providing grid balancing services. Int. J. Hydrog. Energy 2015, 40, 8778–8787.
- Mohammadi, A.; Mehrpooya, M. A comprehensive review on coupling different types of electrolyzer to renewable energy sources. Energy 2018, 158, 632–655.
- Sherif, S.; Barbir, F.; Veziroglu, T. Wind energy and the hydrogen economy—Review of the technology. Solar Energy 2005, 78, 647–660.
- Bellotti, D.; Rivarolo, M.; Magistri, L.; Massardo, A. Thermo-economic comparison of hydrogen and hydro-methane produced from hydroelectric energy for land transportation. Int. J. Hydrog. Energy 2015, 40, 2433–2444.
- Yilmaz, F.; Ozturk, M.; Selbas, R. Thermodynamic performance assessment of ocean thermal energy conversion based hydrogen production and liquefaction process. Int. J. Hydrog. Energy 2018, 43, 10626–10636.
- Khosravi, A.; Syri, S.; Assad, M.; Malekan, M. Thermodynamic and economic analysis of a hybrid ocean thermal energy conversion/photovoltaic system with hydrogen-based energy storage system. Energy 2019, 172, 304–319.
- Balta, M.T.; Hepbasli, A. Potential methods for geothermal-based hydrogen production. Int. J. Hydrog. Energy 2010, 35, 4949–4961.
- Tolga Balta, M.; Dincer, I.; Hepbasli, A. Thermodynamic assessment of geothermal energy use in hydrogen production. Int. J. Hydrog. Energy 2009, 34, 2925–2939.
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrog. Energy 2014, 39, 1–12.
- Mori, D.; Hirose, K. Recent challenges of hydrogen storage technologies for fuel cell vehicles. Int. J. Hydrog. Energy 2009, 34, 4569–4574.
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849.
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611.
- Ramea, K. An integrated quantitative-qualitative study to monitor the utilization and assess the perception of hydrogen fueling stations. Int. J. Hydrog. Energy 2019.
- Santos, D.M.; Sequeira, C.A.; Figueiredo, J.L. Hydrogen production by alkaline water electrolysis. Quimica Nova 2013, 36, 1176–1193.
- Kreuter, W.; Hofmann, H. Electrolysis: The important energy transformer in a world of sustainable energy. Int. J. Hydrog. Energy 1998, 23, 661–666.
- Feng, Q.; Yuan, X.Z.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sour. 2017, 366, 33–55.
- Rashid, M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. Int. J. Eng. Adv. Technol. (IJEAT) 2015, 4, 2249–8958.
- Ursúa, A.; Gandía, L.M.; Sanchis, P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE 2012, 100, 410–426.
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390.
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403.
- Chisholm, G.; Cronin, L. Hydrogen From Water Electrolysis. In Storing Energy: With Special Reference to Renewable Energy Sources; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 315–343.
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934.
- Marshall, A.; Børresen, B.; Hagen, G.; Tsypkin, M.; Tunold, R. Hydrogen production by advanced proton exchange membrane (PEM) water electrolysers-Reduced energy consumption by improved electrocatalysis. Energy 2007, 32, 431–436.
- Al Shakhshir, S.; Cui, X.; Frensch, S.; Kær, S.K. In-situ experimental characterization of the clamping pressure effects on low temperature polymer electrolyte membrane electrolysis. Int. J. Hydrog. Energy 2017, 42, 21597–21606.
- Li, N.; Araya, S.S.; Kær, S.K. The effect of Fe3+ contamination in feed water on proton exchange membrane electrolyzer performance. Int. J. Hydrog. Energy 2019, 44, 12952–12957.
- Slavcheva, E.; Radev, I.; Bliznakov, S.; Topalov, G.; Andreev, P.; Budevski, E. Sputtered iridium oxide films as electrocatalysts for water splitting via PEM electrolysis. Electrochim. Acta 2007, 52, 3889–3894.
- Slavcheva, E.; Borisov, G.; Lefterova, E.; Petkucheva, E.; Boshnakova, I. Ebonex supported iridium as anode catalyst for PEM water electrolysis. Int. J. Hydrog. Energy 2015, 40, 11356–11361.
- Paulose, M.; Mor, G.K.; Varghese, O.K.; Shankar, K.; Grimes, C.A. Visible light photoelectrochemical and water-photoelectrolysis properties of titania nanotube arrays. J. Photochem. Photobiol. Chem. 2006, 178, 8–15.
- Lindquist, S.E.; Fell, C. Fuels—Hydrogen Generators|Photoelectrolysis. Encycl. Electrochem. Power Sources 2009, 369–383.
- Ahmed, M.; Dincer, I. A review on photoelectrochemical hydrogen production systems: Challenges and future directions. Int. J. Hydrog. Energy 2019, 44, 2474–2507.
- Ausfelder, F.; Beilmann, C.; Bertau, M.; Bräuninger, S.; Heinzel, A.; Hoer, R.; Koch, W.; Mahlendorf, F.; Metzelthin, A.; Peuckert, M.; et al. Energy Storage as Part of a Secure Energy Supply. ChemBioEng Rev. 2017, 4, 144–210.
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88.
- Methanol as a Vehicle Fuel | Methanex Corporation. Available online: https://www.methanex.com/about-methanol/methanol-vehicle-fuel (accessed on 10 December 2019).
- Gavaghan, H. Technology: California cleans up its cars with methanol|New Scientist. Available online: https://www.newscientist.com/article/mg12517073-900-technology-california-cleans-up-its-cars-with-methanol/ (accessed on 25 January 2020).
- Fuel Freedom Foundation. When California had 15,000 methanol cars—Fuel Freedom Foundation. Available online: https://www.fuelfreedom.org/when-california-had-15000-methanol-cars/ (accessed on 10 December 2019).
- International Maritime Organization (IMO). Sulphur 2020—Cutting Sulphur Oxide Emissions. Available online: http://www.imo.org/en/MediaCentre/HotTopics/Pages/Sulphur-2020.aspx (accessed on 10 December 2019).
- International Maritime Organization (IMO). Energy efficiency and the reduction of GHG emissions from ships. Available online: http://www.imo.org/en/MediaCentre/HotTopics/GHG/Pages/default.aspx (accessed on 10 December 2019).
- Gregory Dolan. Overview of Global Methanol Fuel Blending; Technical report; Methanol Institute: Alexandria, VA, USA, 2019.
- A.P. Moller—Maersk and Lloyds Register. Alcohol, Biomethane and Ammonia are the Best-Positioned Fuels to Reach Zero Net Emissions. Available online: https://www.maersk.com/news/articles/2019/10/24/alcohol-biomethane-and-ammonia-are-the-best-positioned-fuels-to-reach-zero-net-emissions (accessed on 10 December 2019).
- Transport & Environment (TE). Roadmap to Decarbonising European Aviation; Technical report; European Federation for Transport & Environment AISBL: Brussels, Belgium, 2018.
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989.
- Kamarudin, S.; Achmad, F.; Daud, W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrog. Energy 2009, 34, 6902–6916.
- Icardi, U.; Specchia, S.; Fontana, G.; Saracco, G.; Specchia, V. Compact direct methanol fuel cells for portable application. J. Power Sour. 2008, 176, 460–467.
- Kang, K.; Park, S.; Cho, S.O.; Choi, K.; Ju, H. Development of Lightweight 200-W Direct Methanol Fuel Cell System for Unmanned Aerial Vehicle Applications and Flight Demonstration. Fuel Cells 2014, 14, 694–700.
- Gong, A.; Verstraete, D. Fuel cell propulsion in small fixed-wing unmanned aerial vehicles: Current status and research needs. Int. J. Hydrog. Energy 2017, 42, 21311–21333.
- González-Espasandín, Ó.; Leo, T.J.; Navarro-Arévalo, E. Fuel cells: A real option for unmanned aerial vehicles propulsion. Sci. World J. 2014, 2014.
- SerEnergy A/S. The Benefits of Methanol Fuel Cells. Available online: https://serenergy.com/ (accessed on 10 December 2019).
- Lotrič, A.; Sekavčnik, M.; Hočevar, S. Effectiveness of heat-integrated methanol steam reformer and polymer electrolyte membrane fuel cell stack systems for portable applications. J. Power Sour. 2014, 270, 166–182.
- Ribeirinha, P.; Alves, I.; Vázquez, F.V.; Schuller, G.; Boaventura, M.; Mendes, A. Heat integration of methanol steam reformer with a high-temperature polymeric electrolyte membrane fuel cell. Energy 2017, 120, 468–477.
- Thomas, S.; Araya, S.S.; Frensch, S.H.; Steenberg, T.; Kær, S.K. Hydrogen mass transport resistance changes in a high temperature polymer membrane fuel cell as a function of current density and acid doping. Electrochim. Acta 2019, 317, 521–527.
- Andreasen, S.J.; Kær, S.K.; Justesen, K.K.; Sahlin, S.L. High Temperature PEM Fuel Cell Systems, Control and Diagnostics. In High Temperature Polymer Electrolyte Membrane Fuel Cells; Springer International Publishing: Cham, Switzerland, 2016; pp. 459–486.
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrog. Energy 2016, 41, 21310–21344.
- Andreasen, S.J. Design and Control of High Temperature PEM Fuel Cell System. Ph.D. Thesis, Aalborg University, Aalborg, Denmark, 2009.
- Kurz, T.; Küfner, F.; Gerteisen, D. Heating of Low and High Temperature PEM Fuel Cells with Alternating Current. Fuel Cells 2018, 18, 326–334.
- Geissler, K.; Newson, E.; Vogel, F.; Truong, T.B.; Hottinger, P.; Wokaun, A. Autothermal methanol reforming for hydrogen production in fuel cell applications. Phys. Chem. Chem. Phys. 2001, 3, 289–293.
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sustain. Energy Rev. 2014, 29, 355–368.
- Karim, A.; Bravo, J.; Gorm, D.; Conant, T.; Datye, A. Comparison of wall-coated and packed-bed reactors for steam reforming of methanol. Catal. Today 2005, 110, 86–91.
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–steam reforming on Cu/ZnO/Al2O3. Part 1: the reaction network. Appl. Catal. Gen. 1999, 179, 21–29.
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol-steam reforming on Cu/ZnO/Al2O3 catalysts. Part 2. A comprehensive kinetic model. Appl. Catal. Gen. 1999, 179, 31–49.
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Steady-state isotopic transient kinetic analysis of steam reforming of methanol over Cu-based catalysts. Appl. Catal. Environ. 2009, 88, 490–496.
- Huang, C.Y.; Sun, Y.M.; Chou, C.Y.; Su, C.C. Performance of catalysts CuO–ZnO–Al2O3, CuO–ZnO–Al2O3–Pt–Rh, and Pt-Rh in a small reformer for hydrogen generation. J. Power Sour. 2007, 166, 450–457.
- Purnama, H.; Ressler, T.; Jentoft, R.E.; Soerijanto, H.; Schlögl, R.; Schomäcker, R. CO formation/selectivity for steam reforming of methanol with a commercial CuO/ZnO/Al2O3 catalyst. Appl. Catal. Gen. 2004, 259, 83–94.
- Sahlin, S.L. Characterization and Modeling of a Methanol Reforming Fuel Cell System. Ph.D. Thesis, Aalborg University, Aalborg, Denmark, 2016.
- Simon Araya, S.; Juhl Andreasen, S.; Venstrup Nielsen, H.; Knudsen Kær, S. Investigating the effects of methanol-water vapor mixture on a PBI-based high temperature PEM fuel cell. Int. J. Hydrog. Energy 2012, 37, 18231–18242.
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. Environ. 2010, 99, 43–57.
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021.
- Twigg, M.V.; Spencer, M.S. Deactivation of copper metal catalysts for methanol decomposition, methanol steam reforming and methanol synthesis. Top. Catal. 2003, 22, 191–203.
- Breen, J.P.; Ross, J.R.H. Methanol reforming for fuel-cell applications: development of zirconia-containing Cu–Zn–Al catalysts. Catal. Today 1999, 51, 521–533.
- Valdés-Solís, T.; Marbán, G.; Fuertes, A.B. Nanosized catalysts for the production of hydrogen by methanol steam reforming. Catal. Today 2006, 116, 354–360.
- Agarwal, V.; Patel, S.; Pant, K. H2 production by steam reforming of methanol over Cu/ZnO/Al2O3 catalysts: transient deactivation kinetics modeling. Appl. Catal. Gen. 2005, 279, 155–164.
- Cao, W.; Chen, G.; Li, S.; Quan, Y. Methanol-steam reforming over a ZnO–Cr2O3/CeO2–ZrO2/Al2O3 catalyst. Chem. Eng. J. 2006, 119, 93–98.
- Lee, M.T.; Greif, R.; Grigoropoulos, C.P.; Park, H.G.; Hsu, F.K. Transport in packed-bed and wall-coated steam-methanol reformers. J. Power Sour. 2007, 166, 194–201.
- Saidi, M. Performance assessment and evaluation of catalytic membrane reactor for pure hydrogen production via steam reforming of methanol. Int. J. Hydrog. Energy 2017, 42, 16170–16185.
- Mobile Hybrid Power shows APU with Serenergy HTPEM. Fuel Cells Bull. 2013, 2013, 8.
- Arsalis, A.; Nielsen, M.P.; Kær, S.K. Modeling and off-design performance of a 1kWe HT-PEMFC (high temperature-proton exchange membrane fuel cell)-based residential micro-CHP (combined-heat-and-power) system for Danish single-family households. Energy 2011, 36, 993–1002.
- Liu, Y.; Lehnert, W.; Janßen, H.; Samsun, R.C.; Stolten, D. A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. J. Power Sour. 2016, 311, 91–102.
- First methanol filling station opens to serve range-extender cars. Fuel Cells Bull. 2015, 2015, 7–8.
- Blue World Technologies signs strategic cooperation agreement with Chinese electric vehicle manufacturer AIWAYS—Blue World Technologies. Available online: https://www.blue.world/blue-world-technologies-signs-strategic-cooperation-agreement-with-chinese-electric-vehicle-manufacturer-aiways/ (accessed on 10 December 2019).
- Millo, F.; Caputo, S.; Piu, A. Analysis of a HT-PEMFC range extender for a light duty full electric vehicle (LD-FEV). Int. J. Hydrog. Energy 2016, 41, 16489–16498.
- Blue World Technologies. Available online: https://www.blue.world (accessed on 10 December 2019).
- Gas Fuelled Ships onboard Fuel Cell powered Viking Mariella. Available online: https://www.motorship.com/gfsconference/latest-news101/unique-venue-revealed-another-first-for-gfs!2 (accessed on 10 December 2019).
- Serenergy. The first methanol fuel cell powered vessel in Germany is now sailing the waters of lake Baldeneysee. Available online: https://serenergy.com/the-first-methanol-fuel-cell-powered-vessel-in-germany-is-now-sailing-the-waters-of-lake-baldeneysee/ (accessed on 10 December 2019).
- ZBT, Helbio, Advent demo HTPEM stack in CHP configuration. Fuel Cells Bull. 2013, 2013, 11.
- Nielsen, E.R.; Prag, C.B.; Bachmann, T.M.; Carnicelli, F.; Boyd, E.; Walker, I.; Ruf, L.; Stephens, A. Status on Demonstration of Fuel Cell Based Micro-CHP Units in Europe. Fuel Cells 2019, 19, 340–345.
- Elcore to Install 135 Micro-CHP Fuel Cells in Europe under ene.field Project. Available online: http://www.fuelcelltoday.com/news-archive/2013/august/elcore-to-install-135-micro-chp-fuel-cells-in-europe-under-enefield-project (accessed on 10 December 2019).
- Oh, M.H.; Yoon, Y.S.; Park, S.G. The electrical and physical properties of alternative material bipolar plate for PEM fuel cell system. Electrochim. Acta 2004, 50, 777–780.
- Ribeirinha, P.; Boaventura, M.; Lopes, J.C.B.; Sousa, J.M.; Mendes, A. Study of different designs of methanol steam reformers: Experiment and modeling. Int. J. Hydrog. Energy 2014, 39, 19970–19981.
- Pan, C.; He, R.; Li, Q.; Jensen, J.O.; Bjerrum, N.J.; Hjulmand, H.A.; Jensen, A.B. Integration of high temperature PEM fuel cells with a methanol reformer. J. Power Sour. 2005, 145, 392–398.
- Ji, F.; Yang, L.; Li, Y.; Sun, H.; Sun, G. Performance enhancement by optimizing the reformer for an internal reforming methanol fuel cell. Energy Sci. Eng. 2019, 1–11.