Post-COVID depression affects people who have had SARS-CoV-2 infection. A very important issue for the mental health of the general population is to look for the causes of this complication and its biomarkers. This will help in faster diagnosis and effective treatment of the affected patients.
1. Introduction
The most common symptoms of coronavirus disease are fever, cough, shortness of breath, muscle pain, headache, diarrhea, rhinorrhea, loss of smell and taste [1][2][3][2,3,4]. In addition, there are more and more reports of mental health problems in people who have survived SARS-CoV-2 infection. The most frequently described mental disorders are major depressive disorder (MDD), post-traumatic stress disorder (PTSD), anxiety disorders, obsessive-compulsive disorders (OCD) and insomnia [4][5][6][5,6,7]. These disorders occur mainly in the acute phase of infection and shortly after it [6][7][8][7,8,9]. While the symptoms of PTSD, anxiety disorders and insomnia gradually disappear, it has been shown that symptoms of MDD persist even in the third month of follow-up [6][7].
2. Inflammatory Factors
The role of inflammation and inflammatory factors in the development of MDD is well understood
[9][10][41,42]. It has been proven that patients suffering from inflammatory diseases, such as multiple sclerosis or systemic lupus, who have also been treated with cytokines have a greater chance of developing MDD
[11][12][13][14][43,44,45,46]. COVID-19 is a disease that can cause systemic inflammation and a cytokine storm
[15][47], and is therefore analogous to inflammatory diseases, and may contribute to the development of major depressive disorder (MDD).
Inflammatory cytokines are molecules that mediate the immune response upon activation of the peripheral immune system. Cytokines such as tumor necrosis factor alpha (TNFα), interferon-γ (IFN-γ), interleukin 1 (IL-1) play a role primarily in enhancing the cellular response, while cytokines such as interleukin 6 (IL-6), interleukin 10 (IL-10), interleukin 13 (IL-13) are more associated with the humoral response
[9][16][41,48]. Cytokines can be produced in the brain by astrocytes and microglia
[17][18][49,50] or reach it from the periphery due to several mechanisms such as passage through leaky blood-brain barrier (BBB) regions, including the choroid plexus and periventricular organs, active transport through cytokine transport molecules on the endothelium of the brain, transmission of cytokine signals during an infection in the abdominal cavity via afferent nerve fibers such as the vagus nerve, the passage of activated monocytes into the brain from the periphery, or by signals of second-messengers from the BBB endothelial lining which results in overproduction of cytokines
[19][20][21][51,52,53].
2.1. IL-6/sIL-6R
Interleukin 6 (IL-6) is a pro-inflammatory pleiotropic cytokine secreted mainly by monocytes and macrophages under the influence of interleukin 1β and TNF-α, but also by astrocytes and microglia. It belongs to the family of proteins that use gp130 as a signal transmitter
[22][61].
It is the cytokine most often described in MDD, and the studies describing its involvement in MDD are mostly consistent with each other
[23][24][9][25][26][32,34,41,65,66]. According to some studies, high levels of IL-6 correlate with the severity of MDD symptoms in patients who do not respond to treatment
[27][67]. It has been observed that in patients with MDD, increased levels of IL-6 correlated with attention deficit disorder, and one study showed that its increase was prior to the incidence of cognitive impairment in patients with MDD
[26][66].
High levels of IL-6 is widely described in relation to COVID-19, and its level corresponds to the severity of the disease
[6][28][29][30][31][7,68,69,70,71]. In one study, high levels of sIL-6R were also detected in few COVID-19 (+) patients, but the results are not consistent and more research is needed
[31][71]. Higher levels of IL-6 in tandem with higher levels of sIL-6R lead to increased trans-signaling
[32][72]. Thus, if the severity of COVID-19 correlates with the amount of IL-6, patients with severe course may be at greater risk of developing post-COVID depression.
2.2. IL-10
Interleukin 10 (IL-10) is an anti-inflammatory cytokine produced by Th2, Treg cells and M2 macrophages
[33][73]. In the central nervous system (CNS) it is produced, inter alia, by astroglia and microglia
[34][74]. In the latter, the secretion of IL-10 is augmented by neurotransmitters and damage-associated molecules—glutamate and adenosine respectively
[35][75]. Overall IL-10 production increases with increased levels of IL-6 and TNF-α
[36][37][76,77]. It exerts its function by binding with IL-10 receptor (IL-10R) which consist of two subunits—IL-10R
1 and IL-10R
2. The latter is expressed in most cell types, but IL-10R
1 is mostly restricted to cells of hematopoietic lineage. Due to the myeloid origins, microglia express both subunits of IL-10R. Unexpectedly, resting astrocytes also express IL-10R
1 [35][75]. IL-10 limits neuroinflammation, promotes production of immunosuppressive transforming growth factor β (TGF-β) by astrocytes and reduces astrogliosis in response to the pathogenic factors
[35][75].
Elevated levels of IL-10 are often reported in people with MDD
[38][39][40][78,79,80]. Some researchers also observed that MDD severity is related to increased IL-10
[41][42][81,82]. Two meta-analyzes have shown that IL-10 level decreases with effective antidepressant treatment
[38][43][78,83].
To date, many studies have described an increased level of IL-10 in COVID-19 patients
[44][45][46][84,85,86]. In severe cases, the level of IL-10 was higher than in mild cases, and positively correlated with mortality due to COVID-19
[15][45][47][48][49][47,85,87,88,89]. Thus, high levels of IL-10 during and after SARS-CoV-2 infection may suggest an increased susceptibility to developing MDD in these patients. It may also be a good indicator for monitoring the treatment of post-COVID depression, due to its decline with antidepressant treatment
[38][78].
2.3. TNF-α/sTNFR1, sTNFR2
Tumor necrosis factor α (TNF-α) is a pro-inflammatory cytokine produced by Th1 lymphocytes and M1 macrophages, and by astroglia and microglia in the brain
[23][32]. It is one of the earliest cytokines released following trauma, infection or exposure to lipopolysaccharide (LPS)
[50][90] and a regulator of pro-inflammatory cytokine production. Its high level induces the production of, among others, CRP, IL-6, IL-1β
[50][51][52][90,91,92].
It is found to be one of the most promising markers of major depressive disorder. Its elevated level, along with CRP and IL-6, is most consistently described in studies on MDD biomarkers
[53][54][23][55][24][56][57][9][10][58][20,21,32,33,34,35,36,41,42,93]. It is one of the major cytokines involved in neuroinflammation
[59][94] and acts as an inhibitor of hippocampal neurogenesis
[60][61][95,96], an inducer of apoptosis
[62][63][97,98] and negatively affects synaptogenesis, synaptic plasticity and the structure of synaptic membranes
[64][65][99,100]. It increases the permeability of BBB
[66][54] and significantly affects the production of serotonin through its ability to activate indoleamine 2,3-dioxygenase (IDO)
[67][68][101,102].
Its level decreases with effective treatment of MDD
[69][103], and its persistent concentration indicates treatment-resistant depression (TRD)
[70][71][104,105]. It exerts its effects through the TNF-R1 and TNF-R2 receptors on cell membranes. They can be released into the serum and are elevated in MDD
[72][73][74][106,107,108].
TNF-α is elevated in most COVID-19 patients and correlates with the severity of the disease
[5][6][28][30][75][6,7,68,70,109]. COVID-19 (+) patients requiring intensive care unit (ICU) admission have higher TNF-α levels when compared to the patients who do not require treatment on ICU
[45][76][85,110]. One study also found that while in cases of sepsis and acute respiratory distress syndrome (ARDS) in COVID-19 (−) patients, TNF-α levels normalized rapidly after the primary immune response
[77][78][111,112], it is consistently elevated in COVID-19 (+) patients
[45][85]. This may result in an increased chance of developing post-COVID depression due to longer exposure to the pro-inflammatory effects of TNF-α.
In addition, in one of the studies, an increase in the soluble TNF-α receptors—sTNFR1 and TNFR2 in the serum was observed with the increase in the severity of the disease, and the highest levels were recorded in people who eventually died from COVID-19
[47][87]. However, so far, not many studies have studies described changes in TNF-α receptors levels, so the importance of this biomarker requires further research.
2.4. IL-1β
Interleukin 1β (IL-1β) is another pro-inflammatory cytokine secreted by the same cell types as TNF-α and IL-6, i.e., Th1 lymphocytes and M1 macrophages.
Research on the role of IL-1β in MDD is not consistent. Some studies report a correlation and an increase of this cytokine in depression
[79][80][120,121], although not all researchers have found such a link
[38][81][78,122]. The level of IL-1β increases with the increase in BMI
[82][123] and the number of depressive episodes
[23][32], therefore it may cause inaccuracies in research and difficulties in obtaining uniform results. There have also been studies on the proportionality of the level of IL-1β to the severity of MDD symptoms
[83][124] and the fact that it is a risk factor for TRD
[84][85][125,126].
IL-1β has been reported to be elevated in COVID-19 and correlated with disease symptoms
[86][87][88][127,128,129]. Severe patients have significantly elevated levels
[89][130], and one study found high levels of IL-1β persistent in COVID-19 patients up to 4 weeks after symptom onset—similar to IL-6
[90][131]. However, not all studies have shown an IL-1β elevation among COVID-19 patients
[91][92][132,133], therefore, the importance of this interleukin in post-COVID depression requires more investigation.
2.5. IFN-γ
Interferons are a superfamily of endogenous pleiotropic cytokines that play a large role in the maintenance of homeostasis and defense against infection. Interferon gamma (IFN-γ) is a pro-inflammatory cytokine belonging to the type II interferon family
[93][134]. It is secreted mainly by natural killer cells (NK) and CD4 + T cells and macrophages
[94][135].
Disturbances in IFN-γ levels have been documented among patients suffering from MDD
[95][96][136,137]. Its central or peripheral administration causes symptoms of sickness behavior such as anhedonia, memory and social interaction disorders—the same as seen in MDD
[95][136]. IFN-γ has been shown to activate microglia, which contributes to the development of depression
[97][138]. Moreover, IFN-γ largely activates IDO and contributes to the transition of tryptophan to the kynurenine pathway metabolites which are involved in the pathogenesis of MDD
[97][98][99][100][138,139,140,141].
It has been shown that up to 40% of patients treated with interferon for hepatitis C develop symptoms of depression
[98][101][139,142].
However, reports on the role of IFN-γ are inconsistent. Some studies demonstrated its increase among people with MDD
[99][100][102][140,141,143], and some showed no increase or even a decrease
[12][93][44,134].
For COVID-19, the research also diverges. Many studies have shown an increase in the concentration of IFN-γ
[44][50][51][71][93][98][99][103][104][105][84,90,91,105,134,139,140,144,145,146], however, several studies showed a decrease
[15][106][107][47,147,148]. In some studies, the severity of COVID-19 positively correlated with the level of IFN-γ
[48][49][90][88,89,131]. Due to divergent research results, there is still need for more comprehensive studies on this biomarker.
2.6. CRP
C-reactive protein (CRP) is an acute-phase protein produced by liver cells in response to injury, infection or inflammation. Its production is induced by IL-6, and IL-1 enhances this effect. During inflammatory diseases, its serum concentration increases by a minimum of 25%
[108][149]. Baseline CRP levels may be influenced by factors such as body weight, sex, age, nicotinism, and lipid levels
[109][150].
In the context of COVID-19, an increase in CRP concentration is also often described
[6][46][110][111][112][113][7,86,165,166,167,168], and its concentration, according to some reports, correlates with the severity of COVID-19
[110][111][112][114][115][165,166,167,169,170].
One study reported an association of post-COVID depression with inflammatory biomarkers, where higher CRP was observed in COVID-19 (+) depressed patients than in COVID-19 (+) patients without depression
[116][171]. A separate study reported a decrease in baseline CRP levels in COVID-19 (+) patients in whom the severity of depression symptoms decreased compared to COVID-19 (+) patients in whom CRP and depression symptoms were not significantly reduced
[117][172]. Unlike the previous two, the study by Mazza et al. in the third month of follow-up in patients with post-COVID depression showed no association with CRP
[28][68].
The relationship of CRP to COVID-19 (+) is well documented, but due to the inflammatory nature of the disease and therefore an overall increase in CRP, its utility as a marker of post-COVID depression requires further investigation.
2.7. IL-2/sIL-2R
Interleukin 2 (IL-2) is a pro-inflammatory cytokine produced mainly by CD4 + Th cells and to a lesser extent by T CD8+ and NK cells. It is released mainly in response to an antigen
[118][173]. It exerts its action through IL-2 receptors (IL-2R) present mainly on activated T cells.
Studies of MDD biomarkers in affected patients have shown an increased level of this cytokine as well
[119][120][152,175], and its higher concentration is observed in atypical rather than melancholic depression
[120][175]. The influence of IL-2 on the occurrence of depression symptoms is evidenced by the development of depressive symptoms in people and animals to whom it was administered
[95][121][122][123][136,176,177,178]. Moreover, the concentration of sIL-2R is also elevated in people with MDD
[38][124][125][126][78,151,179,180].
In COVID-19 (+) patients, concentrations of both IL-2 and sIL-2R are higher than in COVID-19 (−) patients, especially in severe patients
[15][47][76][106][127][128][129][47,87,110,147,181,182,183]. Two studies also found an association of increased levels of IL-2
[47][87] and sIL-2R with mortality from COVID-19
[129][183], and in another, a high sIL-2R/lymphocyte ratio proved to be the best indicator of critical disease differentiation
[130][184].
There are no studies describing a direct relationship of IL-2R/sIL-2R with post-COVID depression, but due to the clearly described increase in their levels in people with MDD and in people with COVID-19, it may be a promising marker.
2.8. MCP1/CCL2
Monocyte Chemoattractant Protein-1/Chemokine ligand 2 (MCP-1/CCL2) is a chemokine produced by many types of cells, e.g., endothelium, fibroblasts, macrophages, monocytes, astrocytes and microglia.
Patients with COVID-19 also show an increased level of this chemokine compared to healthy people, and in a large proportion of cases it is more elevated in people with severe disease compared to patients with a mild form
[6][47][76][89][90][7,87,110,130,131]. One study also found that MCP-1/CCL2 elevation positively correlated with mortality from COVID-19
[47][87].
The described pro-depressive effect of MCP-1/CCL2 and its increased level, especially in severe cases of COVID-19, make it relevant to further research this chemokine in terms of the development of post-COVID depression.
2.9. SAA1
Serum amyloid A (SAA1) is an acute phase protein that is mainly produced in the liver as a result of IL-1β and IL-6 action
[131][189]. It affects many aspects of the inflammation cascade. by binding to various receptors such as toll like receptors 2 and 4 (TLR2 and TLR4) and the receptor for advanced glycation end products (RAGE) and CD36
[132][190]. SAA1 activates the secretion of cytokines such as TNF-α, IL-6, IL-8, IL-23, IL-18 and IL-10
[39][79]. TLR2 and TLR4 receptors are present on macrophages, microglia and astrocytes
[39][133][79,191], and their stimulation by SAA1 causes production of inflammatory cytokines, including IL-6 and TNF-α, which may play a role in the development of neuroinflammation, which contributes to the occurrence of depressive symptoms
[134][192].
Compared to healthy subjects, patients with symptoms of MDD have elevated levels of SAA1
[39][135][136][137][79,155,193,194]. Patients admitted to hospital for COVID-19 also show elevated levels of this protein compared to healthy controls
[49][138][139][89,195,196]. Additionally, its high concentration positively correlated with the severity of the disease and mortality due to COVID-19. One study noted that a decrease in SAA1 within two weeks of disease was associated with the prognosis of clinical improvement in patients, while its persistently high concentration was associated with death
[138][195].
The clear association of SAA1 with MDD and its persistent high concentration in severe COVID-19 (+) patients suggests that a closer look at its relationship with post-COVID depression is needed.
2.10. Kynurenine Pathway
Kynurenine is a tryptophan metabolite formed as a result of its transformation under the influence of the enzymes indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO).
In a metabolomic study of COVID-19 (+) patients, tryptophan metabolism was the major disorder detected
[29][69]. In patients infected with SARS-CoV-2, decreased levels of tryptophan and serotonin as well as increased levels of kynurenine, 3-hydroxykynurenine, kynurenic acid and picolinic acid (also one of the metabolites of the kynurenine pathway) were found
[29][104][140][141][69,145,212,213]. One of the reasons for the decreased level of tryptophan in COVID-19 (+) patients may also be its decreased absorption in the gut. SARS-CoV-2 causes the internalization and downregulation of ACE2, which is highly expressed in the intestines
[142][214], and which is needed for the expression of the neutral amino acid transporter in the intestinal lumen—B0AT1
[143][215]. Activation of the kynurenine pathway can be indirectly assessed by the kynurenine to tryptophan ratio, which was significantly higher in COVID-19 (+) patients than in healthy controls and correlated positively with disease severity
[29][140][141][69,212,213].
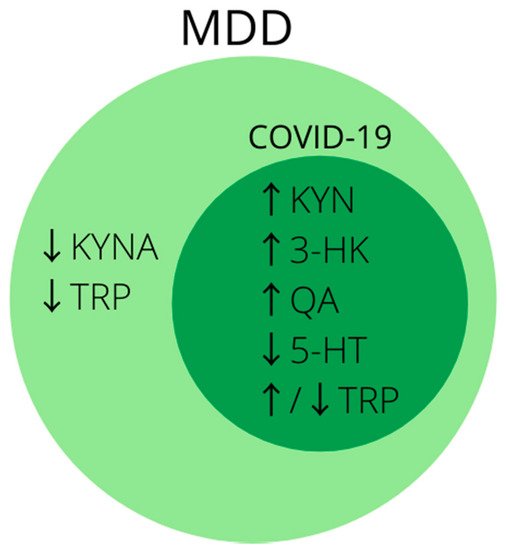
In conclusion, disturbances in tryptophan metabolism and activation of the kynurenine pathway are well described in research on the pathophysiology of MDD and are of great importance in the search for its biomarkers. Analogous changes can be seen in patients infected with SARS-CoV-2 (
Figure 2), and at present disturbances in tryptophan metabolism are one of the most promising theories on the development of post-COVID depression
[144][216]. Therefore, it is worth considering metabolites of the kynurenine pathway in future studies for biomarkers of depression developing in patients after SARS-CoV-2 infection, especially in those with a severe course of the disease.
Figure 2. Summary of the most common biomarkers of activated kynurenine pathway in MDD. Note that biomarkers inside dark-green circle coincide with markers of disturbed tryptophan metabolism in COVID-19. Abbreviations: ↑—increased concentration; ↓—decreased concentration; 3-HK—3-hydroxykynurenine; 5-HT—serotonin; KYN—kynurenine; KYNA—kynurenic acid; MDD—major depressive disorder; QA—quinolinic acid; TRP—tryptophan.
3. Growth Factors
3.1. BDNF
Brain derived neurotrophic factor (BDNF) belongs to the family of neuronal growth factors and its physiological role is to support the differentiation, maturation and survival of dopaminergic, cholinergic and serotonergic neurons of the central nervous system
[145][217]. It also exhibits neuroprotective abilities, is involved in neuroplasticity and enhances long-term potentiation
[146][147][148][218,219,220]. It is produced by neurons as well as by peripheral cells such as leukocytes and endothelial cells and is able to pass through the BBB
[149][221]. Its decreased level is a common finding in people suffering from MDD, and effective antidepressant therapy restores it to normal levels
[149][150][151][221,222,223].
It is proved that ACE2 is associated with a reduction in BDNF levels
[152][224]. It is widely believed that SARS-CoV-2, by using ACE2 to enter cells, causes its downregulation
[153][225]. This mechanism may cause a secondary reduction in BDNF levels. The confirmation of this theory may be reflected in one of the studies performed, in which patients suffering from COVID-19 were tested for serum BDNF levels. The researchers demonstrated that patients with moderate and severe disease have lower BDNF levels than those with mild disease, and during patients’ recovery, their levels returned to normal
[110][165].
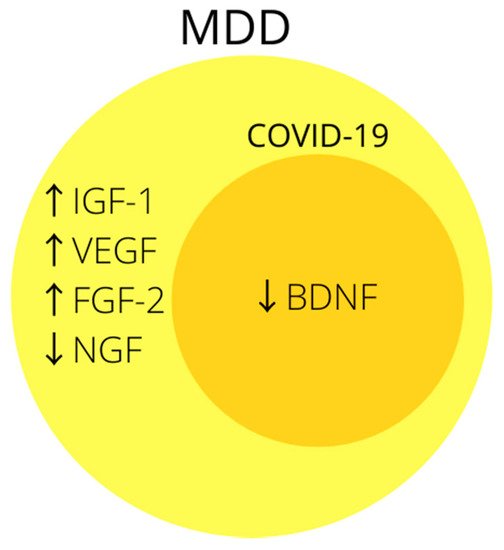
The association of growth factors, especially BDNF, with MDD is often indicated in the literature (Figure 3), and the likely mechanism by which SARS-CoV-2 could reduce it may prove it to be a good biomarker of post-COVID depression. Unfortunately, so far only one study mentions the relationship between BDNF and COVID-19, but its results are promising and it is worth doing more research in this direction.
Figure 3. Summary of the most common changes in growth factors in MDD. Note that biomarkers inside dark-yellow circle coincide with changes in growth factors in COVID-19. Abbreviations: ↑—increased concentration; ↓—decreased concentration; BDNF—brain derived neurotrophic factor; FGF-2—fibroblast growth factor-2; IGF-1—insulin-like growth factor-1; NGF—nerve growth factor; VEGF—vascular endothelial growth factor.